Application of P glycoprotein to influence of INS-1 832/13 cell insulin secretion
A technology of INS-18721 and insulin secretion, which is applied in the field of P-glycoprotein, can solve problems such as unclear influence of insulin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Construction of P-glycoprotein overexpressed insulinoma cells INS-1 832 / 13 cells.
[0022] Prepare the full cell culture medium and common equipment for cell culture and place them in the sterile environment of the cell room. Remove the cell culture medium and gently wash the cells twice with sterile PBS. Add 5mL of cell culture medium, and blow the cells with a 3mL pipette until the wall of the bottle is clear, that is, the cells at the bottom of the bottle have been blown off. Move the suspension to a centrifuge tube, add an appropriate amount of medium, and gently blow and mix the suspension with an electric gun. Move the cell suspension to a centrifuge tube, add an appropriate amount of medium, and then blow with a 10ml pipette gun for 10 minutes and mix well, then spread the suspension on a 6-well plate, grow for 12-24 hours, and wait for the cells to be placed in a logarithmic During the growth period, replace with serum-free medium, and then use diff...
Embodiment 2
[0023] Example 2: Cell viability was detected by MTT method to determine the appropriate concentration of adenovirus and the optimal infection time of adenovirus.
[0024] Cells into a cell suspension, add 100 μL of cell suspension to each well of a 96-well plate, adjust the cell density to 5×10 3 After every 3-5 columns, mix the cell suspension in the "8" pattern, and set up corresponding control wells, that is, only the cell culture medium, MTT, and DMSO groups, and the edge wells of the 96-well plate are used Fill with sterile PBS to rule out edge effects. After 24 hours, different concentrations of adenovirus were added, and 6 replicate wells with 5 gradients were set up. After adenovirus infection, observe the state of the cells under a microscope. Add 10 μL of 0.5% MTT solution (5 mg / ml) to each well, continue to incubate for 4 hours in the dark, and then terminate the MTT incubation, and add 150 μL DMSO to each well, shake on a shaker at low speed for 10 minutes in th...
Embodiment 3
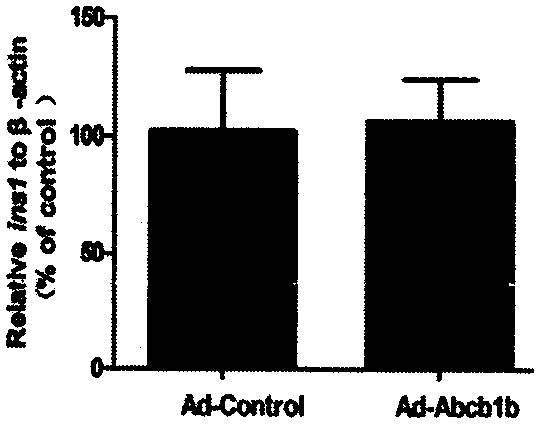
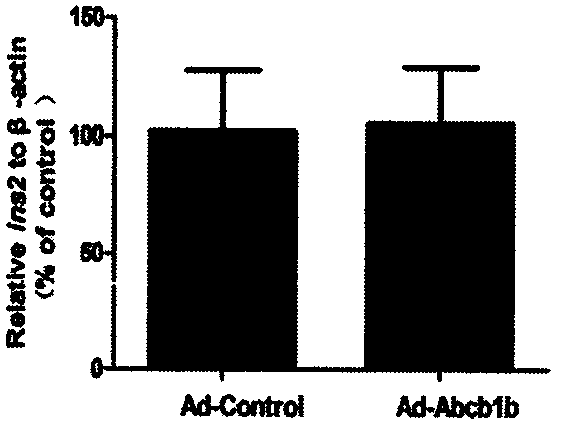
[0025] After embodiment 3P-gp is overexpressed, the efflux pump function of P-gp is significantly enhanced
[0026] After culturing the cells in a twelve-well plate for 24 hours, use 50×10 6 The cells were incubated with Ad-Control and Ad-Abcb1b adenoviruses at pfu / mL concentration, and after the infection, the cells were washed twice with preheated serum-free 1640 culture medium. Add 1 mL of serum-free 1640 culture solution containing 1 μl rhodamine stock solution (1 μg / μl) to each well, and keep the incubator away from light for 3 hours. After incubation, the cells were washed twice with serum-free 1640 to extract cell proteins. Add 220 μL of strong RIPA lysate to each well for lysis for 30 minutes, centrifuge at 12000 r / min for 10 minutes, take the supernatant and add it to a white 96-well plate dedicated to the fluorescence intensity in the dark, add 100 μL of the supernatant to each well; then add rhodamine-prepared For the standard product, under the conditions of λex=...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com