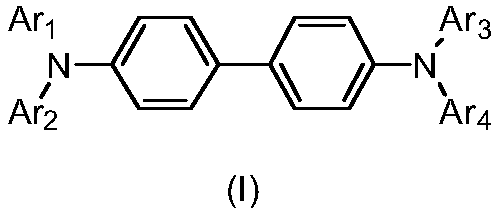
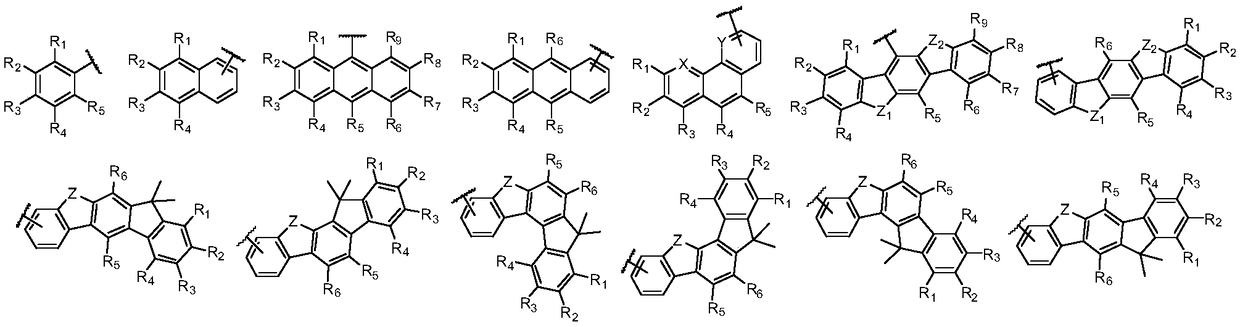
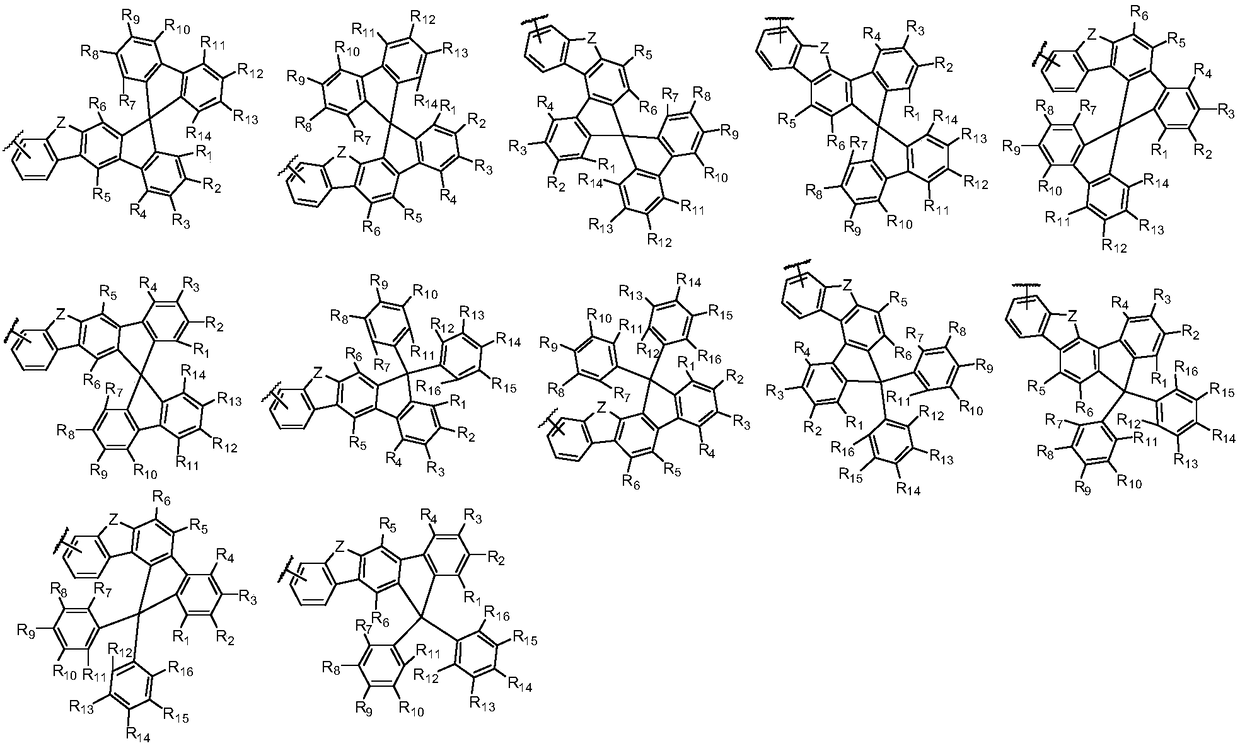
Arylamine derivative and organic electroluminescent device thereof
An aromatic amine derivative, electromechanical technology, applied in electric solid devices, electrical components, organic chemistry, etc., can solve the problems of OLED device color purity, low luminous efficiency, low molecular orbital energy level, and shortened service life, etc. Achieve the effect of improving luminous efficiency and color purity, good film formation and stability, and improving performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] The preparation method of aromatic amine derivatives described in the present invention can be prepared through the following synthetic route:
[0080]
[0081] Among them, Ar 1 、Ar 2 、Ar 3 、Ar 4 as above.
[0082] (1) Using 4-iodo-4'-bromobiphenyl (compound A) as raw material, and containing Ar at the same time 1 and Ar 2 The Buchwald-Hartwig coupling reaction of the arylamine produces intermediate (B);
[0083] (2) intermediate (B) and containing Ar 3 The aromatic amine compound of the same by Buchwald-Hartwig coupling reaction, obtains intermediate (C);
[0084] (3) Finally, intermediate (C) and containing Ar 4 Buchwald-Hartwig coupling reaction of the bromide to obtain the target compound (I).
[0085] The present invention has no special limitation on the reaction conditions of the above-mentioned reactions, and the reaction conditions well-known to those skilled in the art can be adopted. The preparation method is simple and the raw materials are readi...
Embodiment 1
[0091] Embodiment 1: the preparation of intermediate B
[0092] Preparation of Intermediate B-1:
[0093]
[0094] Under an argon atmosphere, 17.95g (50mmol) of compound (A), 16.07g (50mmol) of bis(4-biphenyl)amine, and 9.60g (100mmol) of sodium tert-butoxide were dissolved in 500ml of dehydrated toluene, and stirred Then, 0.23 g (1 mmol) of palladium acetate and 0.20 g (1 mmol) of triphenylphosphine were added, the temperature was raised to 80° C., and the reaction was carried out for 8 hours. After the reaction was finished, cool and filter with a diatomaceous earth / silica gel funnel, and the filtrate was distilled under reduced pressure to remove the solvent, and the residue obtained was recrystallized in toluene and dried to obtain 22.93g (41.5mmol) of the intermediate (B-1 ), the yield is 83%.
Embodiment 2
[0095] Embodiment 2: the preparation of compound HT47
[0096]
[0097] (1) Under an argon atmosphere, dissolve 22.10g (40mmol) of intermediate B-1, 5.73g (40mmol) of 1-naphthylamine (compound M-1), and 7.69g (80mmol) of sodium tert-butoxide in 400ml of dehydrated toluene , add 0.18g (0.8mmol) of palladium acetate and 0.16g (0.8mmol) of triphenylphosphine under stirring, and react at 80°C for 8 hours. After cooling, filter through a diatomaceous earth / silica gel funnel, and the filtrate removes the organic solvent by distillation under reduced pressure, and the resulting residue is recrystallized in toluene and dried to obtain 21.64g (35.2mmol) of intermediate C-1 with a yield of 88 %.
[0098] (2) Under an argon atmosphere, 18.47g (30mmol) of intermediate C-1, 10.90g (30mmol) of bromide N-1, 5.77g (60mmol) of sodium tert-butoxide were dissolved in 300ml of dehydrated toluene, 0.14 g (0.6 mmol) of palladium acetate and 0.12 g (0.6 mmol) of triphenylphosphine were added unde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com