Antimicrobial composition
An antibacterial composition and a technology for antibacterial effect, applied in the field of antibacterial compositions, can solve the problems of not showing the proliferation inhibition effect of MRSA, Pseudomonas aeruginosa or non-tuberculous mycobacteria, and achieve the cure of respiratory tract infection, infection prevention and symptom improvement. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
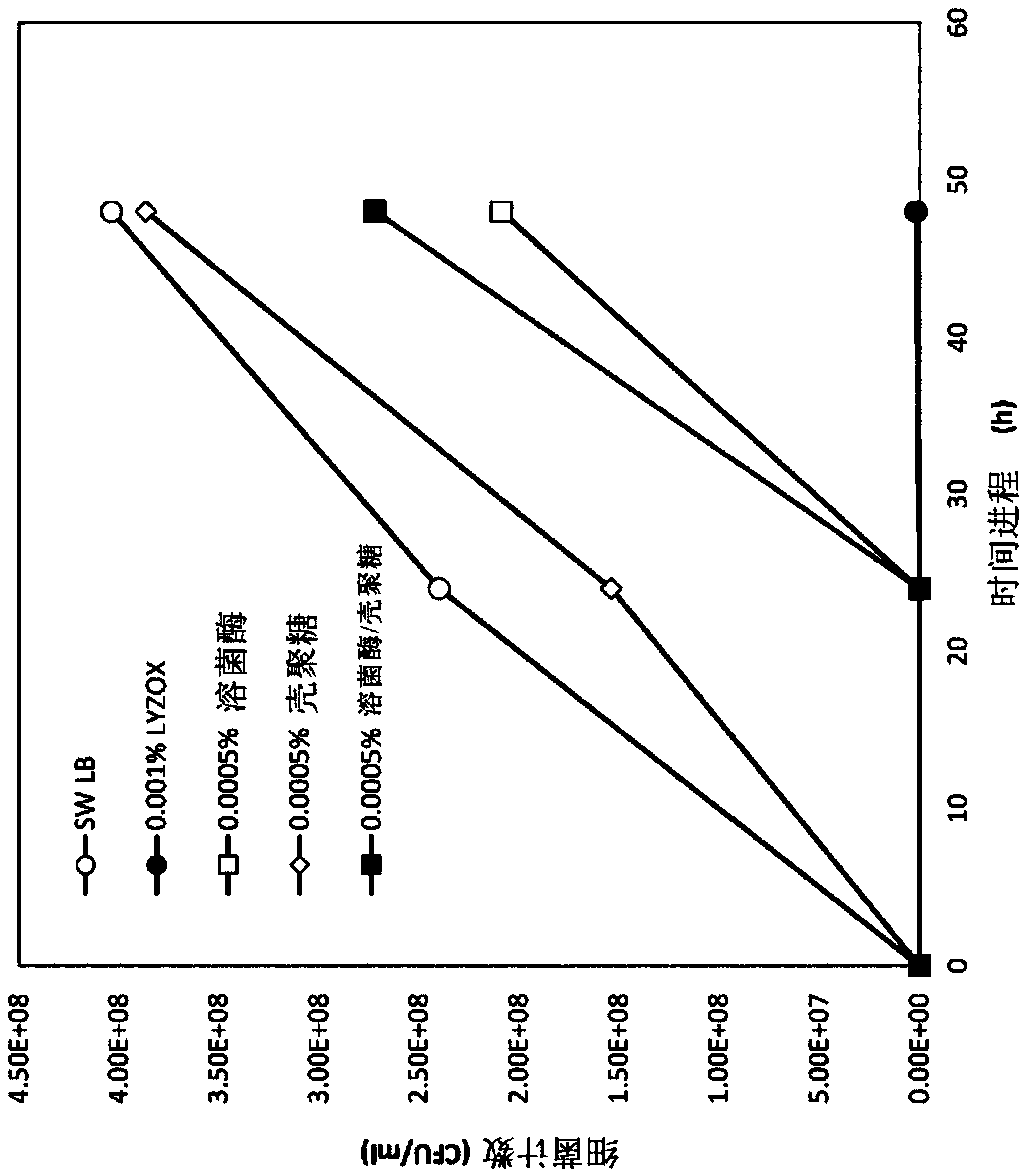
[0047] Proliferation inhibitory effect of lysozyme-chitosan complex, lysozyme alone, chitosan alone and mixture of lysozyme and chitosan on MRSA in diluted medium
[0048] In this test, MRSA IID1677 was used and the strain was cultured at 37° C. for 20 hours in a plain broth medium. The cultured bacterial solution was recovered by centrifugation (3000 rpm, 10 minutes), and suspended in sterilized water. Then, it was diluted with sterilized water so that the absorbance OD600=1.0. Dilute it 2000 times (equivalent to 10 6 CFU / ml), as the reaction bacteria solution.
[0049] 100 μl of the reaction bacteria liquid was added to each LB test medium sample, and shaking culture was performed at 37° C. for 48 hours. Here, each LB test medium sample was prepared by adding the following substances to 10 ml of 1 / 2.5 LB medium.
[0050] (1) 10ml of sterilized water,
[0051] (2) 10 ml of lysozyme-chitosan complex with a concentration of 0.002% (concentration after addition: 0.001%),
...
Embodiment 2
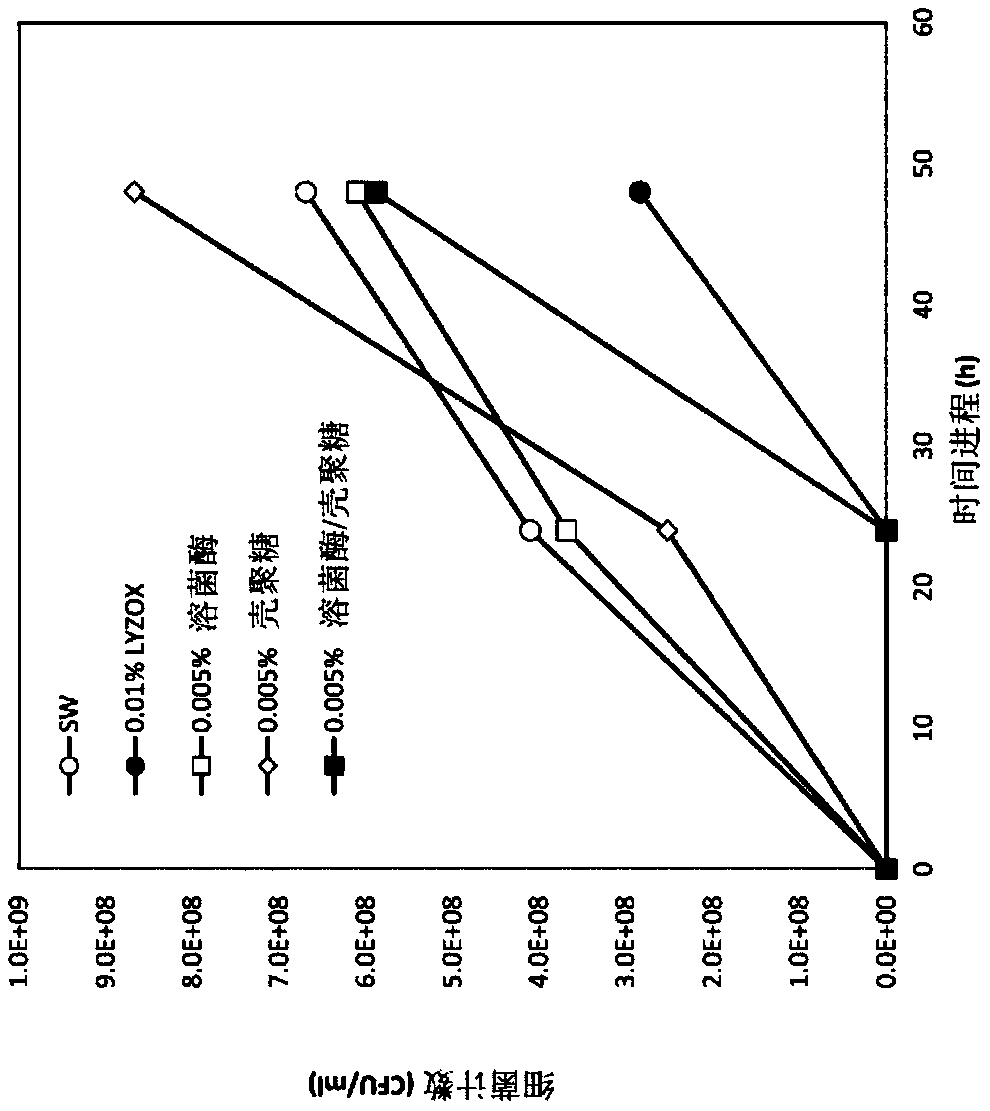
[0059] Inhibitory effect of lysozyme-chitosan complex, lysozyme alone, chitosan alone and mixture of lysozyme and chitosan on the proliferation of Pseudomonas aeruginosa in diluted medium
[0060] In this test, Pseudomonas aeruginosa NBRC13275 was used, and the strain was cultured at 37° C. for 20 hours using a common broth medium. The cultured bacterial solution was recovered by centrifugation (3000 rpm, 10 minutes), and suspended in sterilized water. Then, it was diluted with sterilized water so that the absorbance OD600=1.0. Dilute it 2000 times (equivalent to 10 6 CFU / ml), as the reaction bacteria solution.
[0061] 100 μl of the reaction bacteria liquid was added to each TSB test medium sample, and shake cultured at 37° C. for 48 hours. Here, each TSB test medium sample was prepared by adding 200 μl of 1M phosphate buffer (pH 7.0) to 10 ml of 1 / 2.5 TSB medium, and further adding the following substances.
[0062] (1) 10ml of sterilized water,
[0063] (2) 10 ml of ly...
Embodiment 3
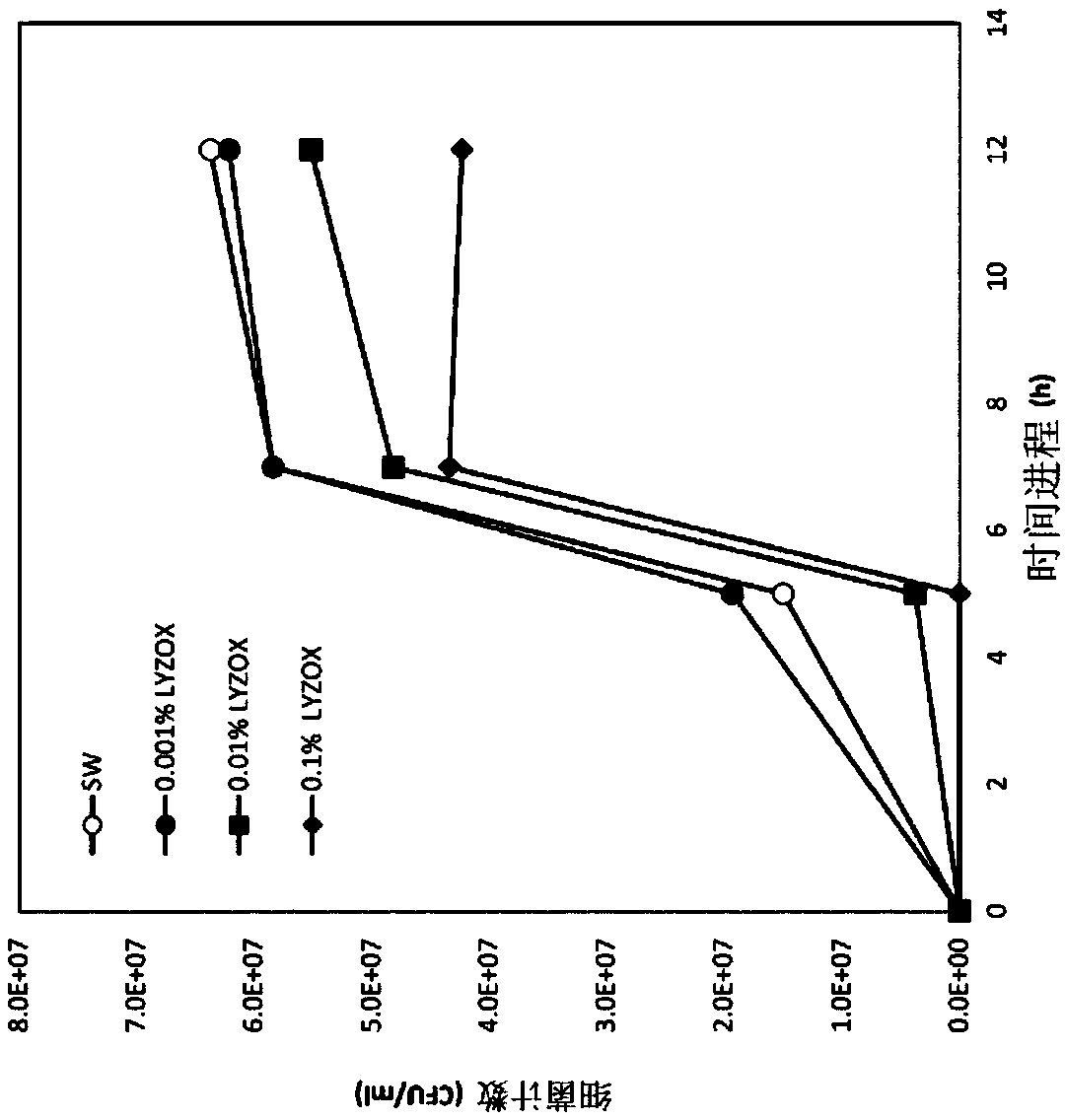
[0070] Proliferation inhibitory effect of lysozyme-chitosan complex, lysozyme alone, chitosan alone and mixture of lysozyme and chitosan on lung MAC in diluted medium
[0071] In this experiment, Mycobacterium avium Chester JCM15429 was used and the strain was cultured at 37° C. for 2 weeks using Middlebrook 7H9 medium. The cultured bacterial solution was recovered by centrifugation (3000 rpm, 10 minutes), and suspended in sterilized water. Then, it was diluted with sterilized water so that the absorbance OD600=1.0. Dilute it 2000 times (equivalent to 10 6 CFU / ml), as the reaction bacteria solution.
[0072] 100 μl of reaction bacteria solution was added to each Middlebrook 7H9 test medium sample, and cultured statically at 37° C. Here, each Middlebrook 7H9 test medium sample was prepared by adding the following substances to 10 ml of 1 / 2.5 Middlebrook 7H9 medium.
[0073] (1) 10ml of sterilized water,
[0074] (2) 10 ml of lysozyme-chitosan complex with a concentration o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com