Preparation and application of fluorescence probes used for real-time sensitive hypochlorous acid detection
A preparation, sulfonic acid group technology, applied in the field of hypochlorous acid fluorescent probes, can solve the problems of complex synthesis, poor selectivity, low sensitivity and the like, and achieve the effects of simple synthesis, good stability and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] (Scheme 1) Dissolve 345mg (1mmol) of 4-chloro-1,8-naphthalimide and 122mg (2mmol) of monoethanolamine in 15mL of ethylene glycol methyl ether and heat to reflux for 10h, and use a dichloromethane / methanol system for column After chromatographic separation, 269 mg of a yellow solid was obtained with a yield of 73%.
[0036] (Scheme 2) Dissolve 345mg (1mmol) of 4-chloro-1,8-naphthalimide and 183mg (3mmol) of monoethanolamine in 15mL of ethylene glycol methyl ether and heat to reflux for 10h, and use a dichloromethane / methanol system for column After chromatographic separation, 299 mg of a yellow solid was obtained with a yield of 81%.
[0037] (Scheme 3) Dissolve 345mg (1mmol) of 4-chloro-1,8-naphthoimide and 305mg (5mmol) of monoethanolamine in 15mL of ethylene glycol methyl ether and heat to reflux for 10h, using a dichloromethane / methanol system for column After chromatographic separation, 339 mg of a yellow solid was obtained with a yield of 92%.
[0038...
Embodiment 2
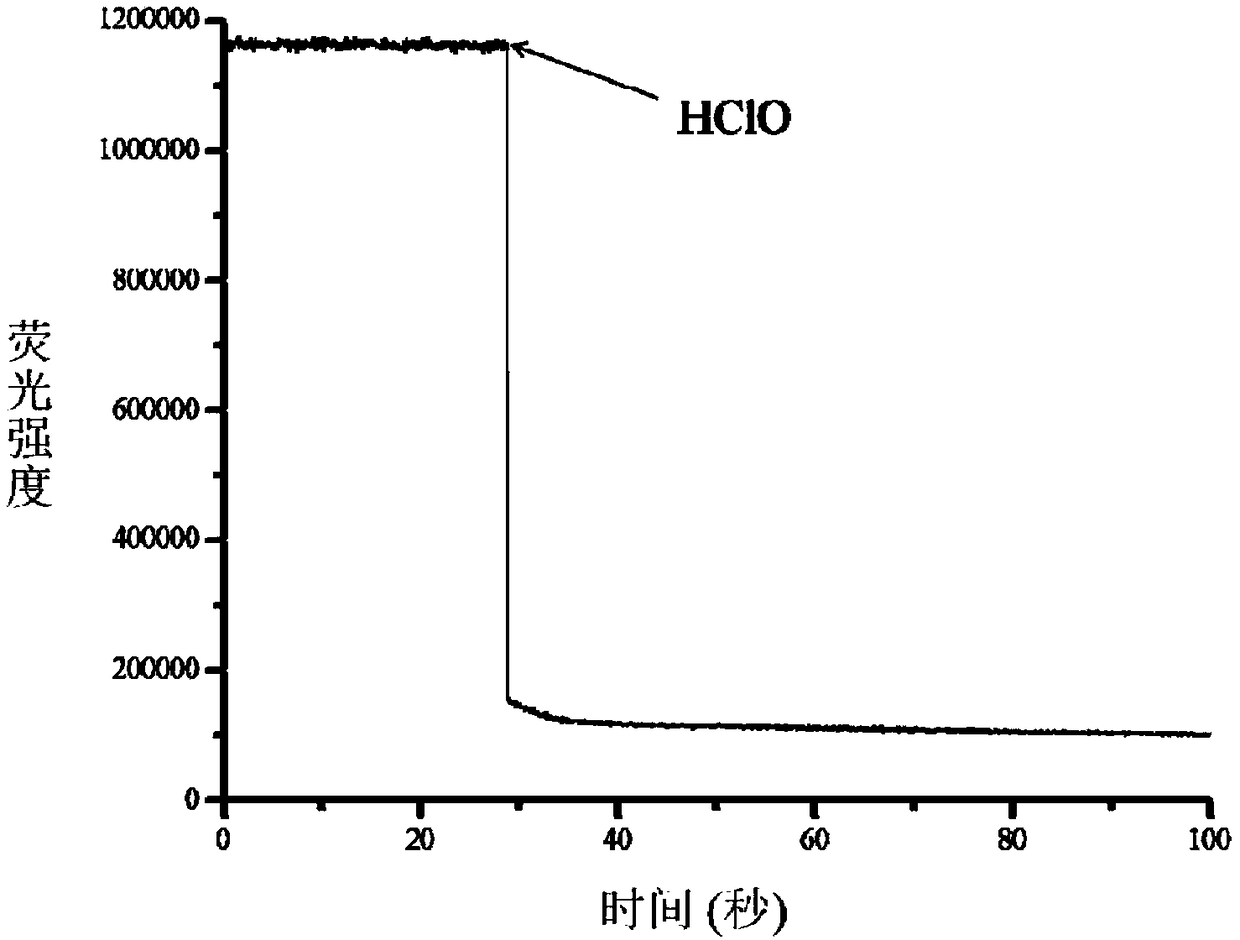
[0041] Test results of probe (5 μM) response time to HClO (5 μM). The above determinations were carried out in 5 mM PBS, pH 7.4 aqueous solution, the probe used was the probe prepared in Example 1, and all spectral tests were measured at 25°C. See results figure 1 .
[0042] from figure 1 It can be seen that after adding 5 μM hypochlorous acid, the fluorescence intensity drops suddenly, and the response can be completed within 3s.
Embodiment 3
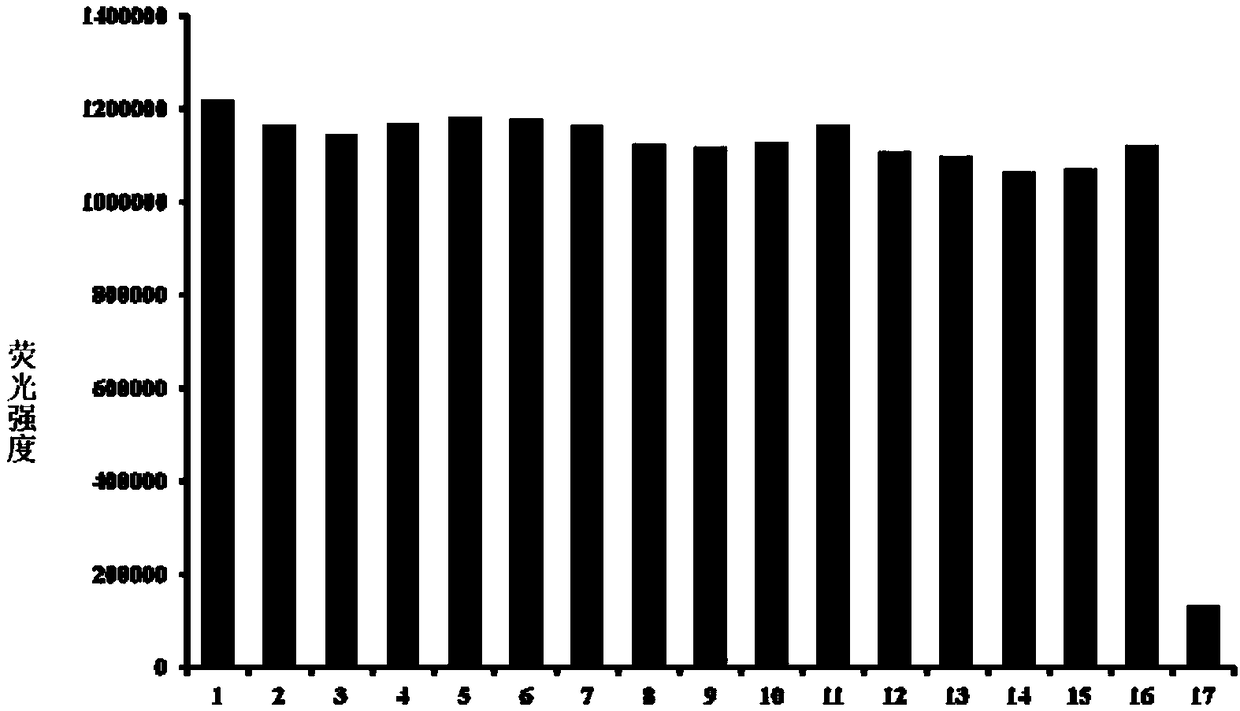
[0044] Effects of different concentrations of hypochlorous acid (0-10 μM) on the fluorescence spectrum of the probe (5 μM). The above determinations were carried out in 5mM PBS, pH 7.4 aqueous solution, the probe used was the probe prepared in Example 1, and all spectral tests were measured after acting at 25°C for 1 min. See results figure 2 a.
[0045] from figure 2b It can be seen that with the increase of the concentration of hypochlorous acid in the probe solution, the fluorescence spectrum gradually weakens, and within the concentration range of 0-1 μM hypochlorous acid, the concentration of hypochlorous acid and the fluorescence intensity show a linear relationship, and the detection The output limit is as low as 2.3nM. Therefore, the probe of the present invention can more accurately determine the content of hypochlorous acid in the sample to be tested.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com