Method using biological method to prepare raspberry ketone
A technology of raspberry ketone and biological method is applied in the field of bioengineering, and can solve the problems of relying on the supply of anisaldehyde raw materials, and achieve the effects of increasing yield and increasing conversion efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
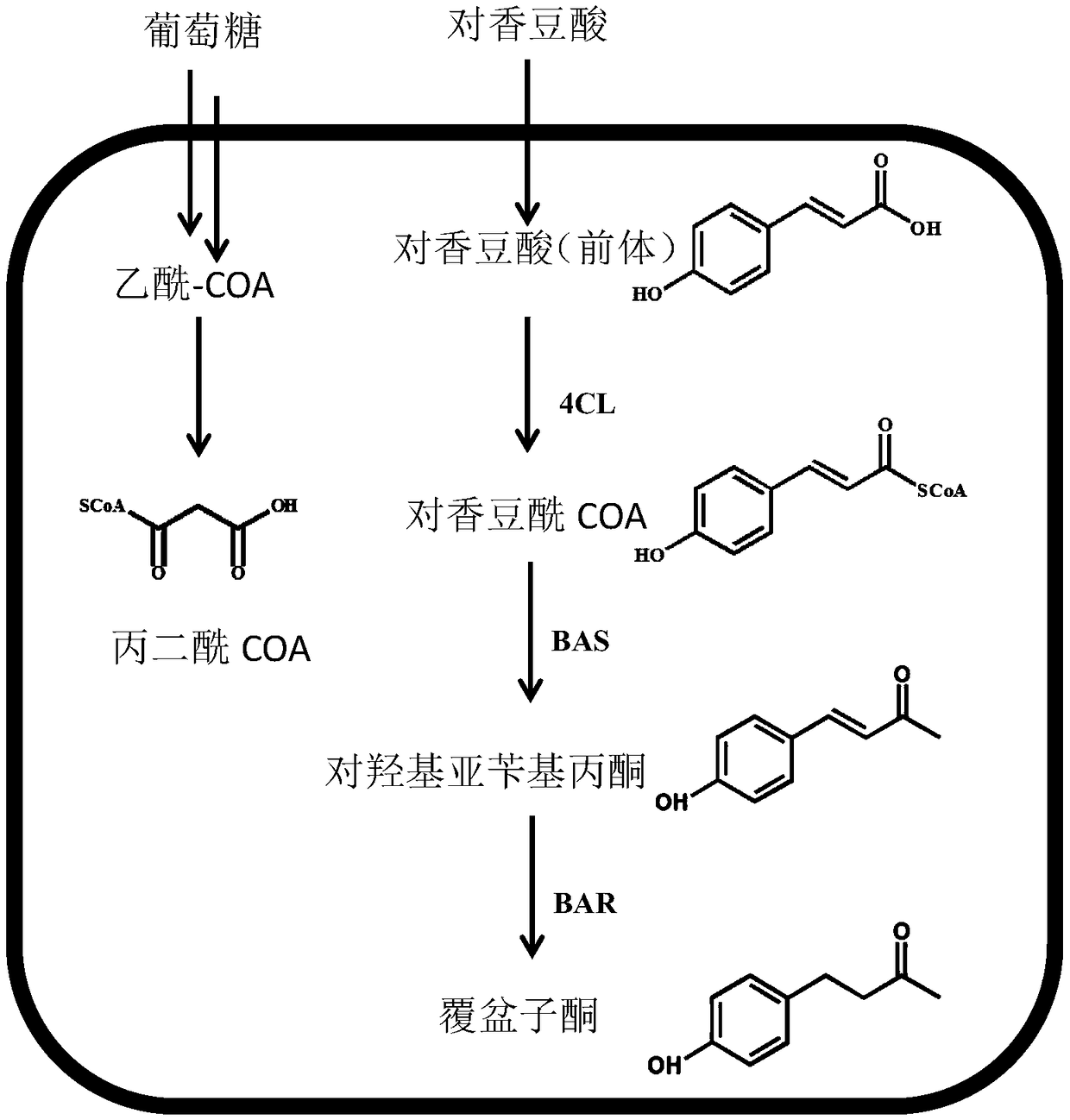
[0032] The nucleotide sequence of the gene 4CL encoding the 4-coumaroyl-CoA ligase is shown in SEQ ID NO.1, which is derived from the plant parsley (Petroselinum crispum) and codon-optimized; encoding the benzyl The nucleotide sequence of the gene BAS of acetone synthase is shown in SEQ ID NO.2, derived from the plant Rheum palmatum (Rheum palmatum) and through codon optimization; the nucleoside of the gene RiRZS1 encoding the benzyl acetone reductase The acid sequence is shown as SEQ ID NO.3, which is derived from the plant Raspberry ketone and has been codon optimized.
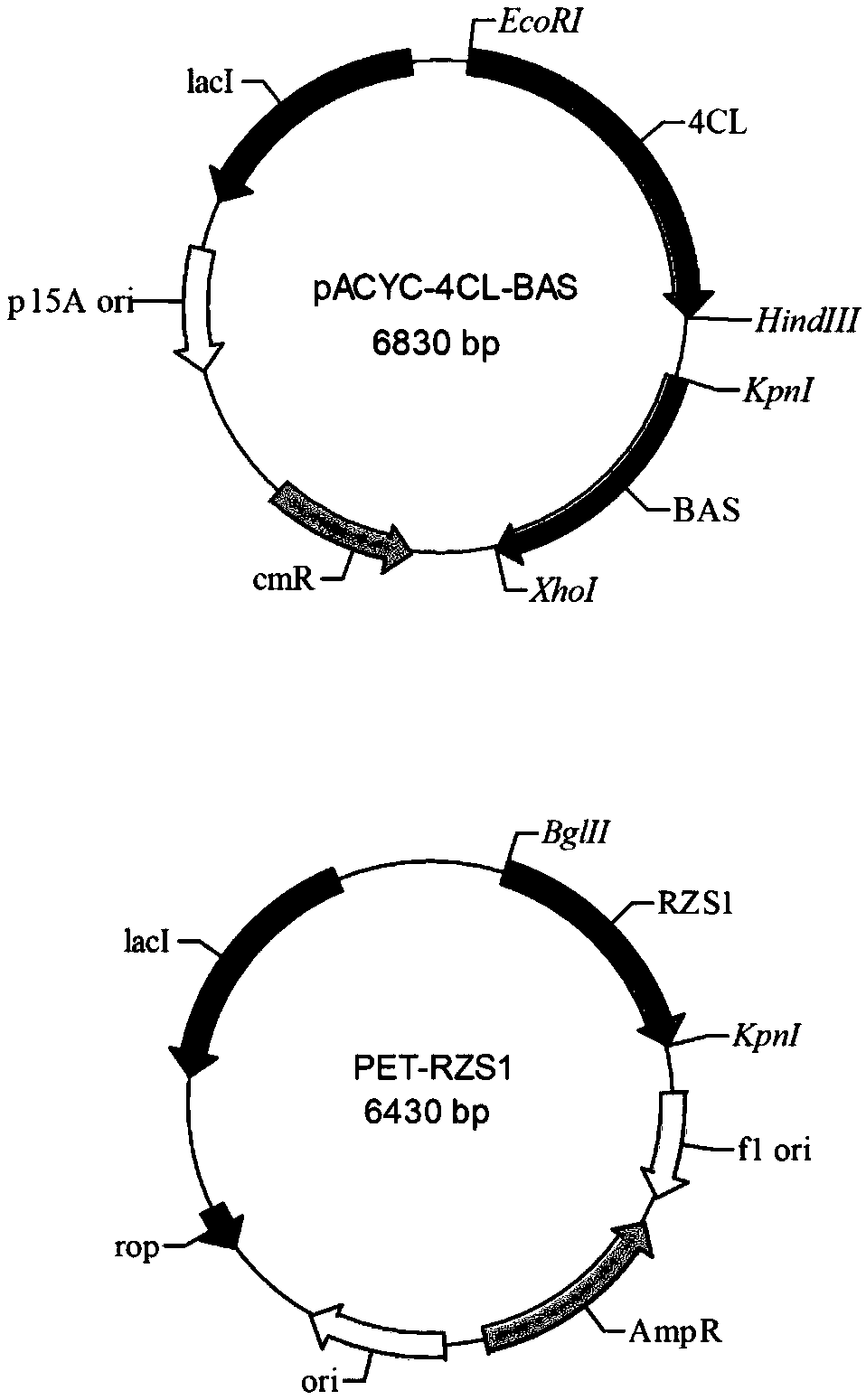
[0033] Construction of plasmid pACYC-BAS-4CL:
[0034]Use primers A1 (SEQ ID No.4) and A2 (SEQ ID No.5) to clone gene 4CL2 (SEQ ID NO.1 encoding 4-coumaroyl-CoA ligase) by PCR: use primer B1 (SEQ ID No. 5) by PCR No.6) and B2 (SEQ ID No.7) were cloned to obtain the gene BAS (SEQ ID NO.2 encoding benzylacetone synthase). First, the recombinant plasmid pACYC-4CL was obtained by one-step cloning with EcoRI an...
Embodiment 2
[0044] Construction of plasmid pET-RZS1:
[0045] Select pET-Duet-1 as the expression vector of RiRZS1 gene. The gene RiRZS1 (SEQ ID NO.3 encoding benzylacetone reductase) was cloned by PCR using primers C1 (SEQ ID NO.8) and C2 (SEQ ID NO.9). After the vector pET-Duet-1 was digested with BglII and KpnI restriction endonucleases, the RiRZS1 gene was inserted into the corresponding restriction site of the plasmid pET-Duet-1 by a one-step cloning method to obtain the recombinant plasmid pET-RZS1 . The PCR reaction conditions were as follows: 95°C for 5 min; 98°C for 10 s, 55°C for 5 s, 72°C for 5 s / kb (30 cycles); 72°C for 10 min, and then verified by 1% agarose gel electrophoresis and recovered the PCR amplification product. One-step cloning specific method Mix fragment (0.04-0.08ng / bp) and vector (0.005-0.01ng / bp) in the same PCR tube, add 2-4μl of recombinase buffer and 1-2μl of recombinase, add ddH2O Make up to 10-20 μl and react at 37°C for 30 minutes to obtain the recomb...
Embodiment 3
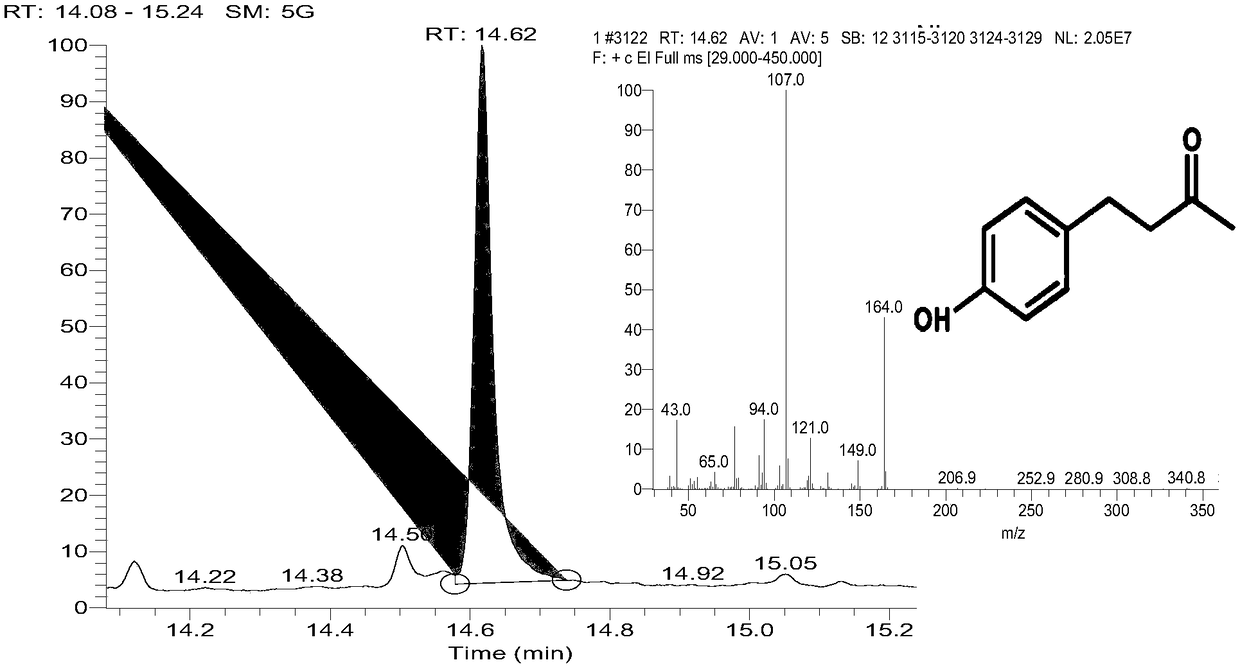
[0051] Construction of raspberry ketone producing strain Mu1:
[0052] Plasmids pACYC-BAS-4CL2 and pET-RZS1 were simultaneously introduced into Escherichia coli BL21(DE3) by heat shock method, and the resulting positive strain was named Mu1. The antibiotics in the medium were chloramphenicol (25-50mg / mL) and ampicillin (50-100mg / mL). Among them, the basic operation of thermal shock method:
[0053] 1) Thaw the Escherichia coli competent cells stored at -80°C in ice for 10 minutes;
[0054] 2) Add 0.1ng-10ng of the corresponding plasmid to E. coli competent cells, mix gently and place in ice for 30min;
[0055] 3) Heat shock in a water bath at 42°C for 45-90 seconds, and immediately place it in ice for 1-2 minutes;
[0056] 4) Add 890 μl of LB medium;
[0057] 5) Place in a shaker at 37°C, 180rmp-300rmp, and incubate for 1 hour;
[0058] 6) Take an appropriate amount of bacterial solution to coat the plate, and place the plate upside down in a 37°C incubator overnight;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com