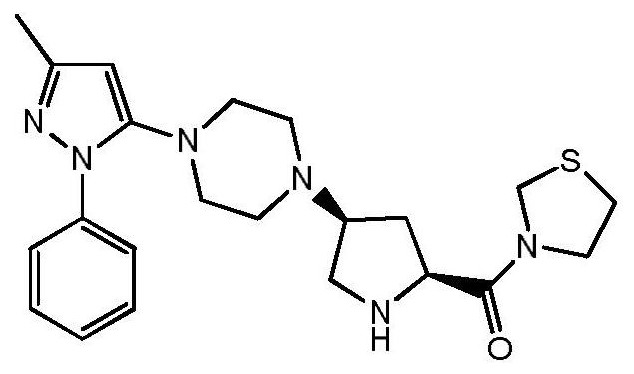
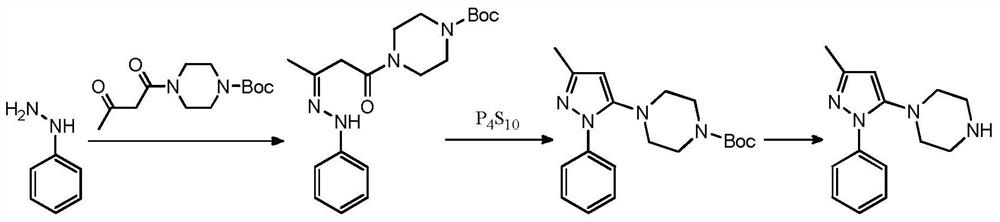
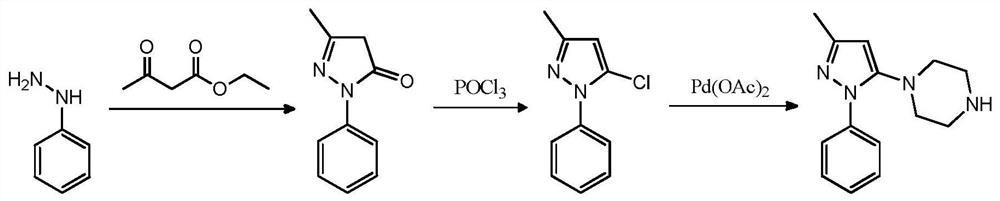
A kind of safe preparation method of 1-(3-methyl-1-phenyl-1h-pyrazol-5-yl)piperazine
A technology of methyl and pyrazole, applied in the field of safe preparation of 1-piperazine, can solve problems affecting product yield and purity, potential safety hazards, strong explosiveness, etc., and achieve avoidance of unsafe reagents, high safety, The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]1) Add 3.5g (0.028mol) N-acetylpiperazine and 20mL toluene to a 50mL single-mouth bottle equipped with a water separator, stir and heat to reflux, after the water removal is completed, cool to room temperature, add 20mL DMF, 4g anhydrous Potassium carbonate, 5.5g (0.025mol) raw materials, heat up and distill toluene out at the same time, react at a temperature of 130°C for 6h, after the reaction, cool to room temperature, filter, rinse the filter cake (5mL*2) with DMF, concentrate the filtrate, and the residue Add water and 1,2-dichloroethane for extraction, and concentrate to obtain an oil.
[0044] 2) Add the oil obtained in the previous step and 20 mL of n-butanol to a 50 mL single-mouth bottle, stir evenly, add 5 mL of hydrochloric acid dropwise, and heat up to reflux for 3 hours. After the reaction, cool to room temperature, add 20 mL of water and stir thoroughly, ethyl acetate Wash the water layer (10mL*2), separate the water layer, adjust the pH of the water layer...
Embodiment 2
[0046] 1) Add 5.3g (0.028mol) N-benzoylpiperazine and 20mL toluene to a 50mL single-mouth bottle equipped with a water separator, stir and heat to reflux, after the water removal is completed, cool to room temperature, add 20mL DMF, 4g Anhydrous potassium carbonate, 5.5g (0.025mol) raw materials, heat up and distill toluene out at the same time, react at a temperature of 130°C for 6h, after the reaction is completed, cool to room temperature, filter, rinse the filter cake (5mL*2) with DMF, concentrate the filtrate, The residue was extracted with water and 1,2-dichloroethane, and concentrated to obtain an oil.
[0047] 2) Add the oil obtained in the previous step and 20 mL of n-butanol to a 50 mL single-mouth bottle, stir evenly, add 5 mL of hydrochloric acid dropwise, and heat up to reflux for 3 hours. After the reaction, cool to room temperature, add 20 mL of water and stir thoroughly, ethyl acetate Wash the water layer (10mL*2), separate the water layer, adjust the pH of the...
Embodiment 3
[0049] 1) Add 3.5g (0.028mol) N-acetylpiperazine and 20mL toluene to a 50mL single-mouth bottle equipped with a water separator, stir and heat to reflux, after the water removal is completed, cool to room temperature, add 20mL DMSO, 4g anhydrous Potassium carbonate, 5.5g (0.025mol) raw materials, heat up and distill toluene out at the same time, react at a temperature of 130°C for 6h, after the reaction, cool to room temperature, filter, rinse the filter cake (5mL*2) with DMSO, concentrate the filtrate, and the residue Add water and 1,2-dichloroethane for extraction, and concentrate to obtain an oil.
[0050] 2) Add the oil obtained in the previous step and 20 mL of n-butanol to a 50 mL single-mouth bottle, stir evenly, add 5 mL of hydrochloric acid dropwise, and heat up to reflux for 3 hours. After the reaction, cool to room temperature, add 20 mL of water and stir thoroughly, ethyl acetate Wash the water layer (10mL*2), separate the water layer, adjust the pH of the water la...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com