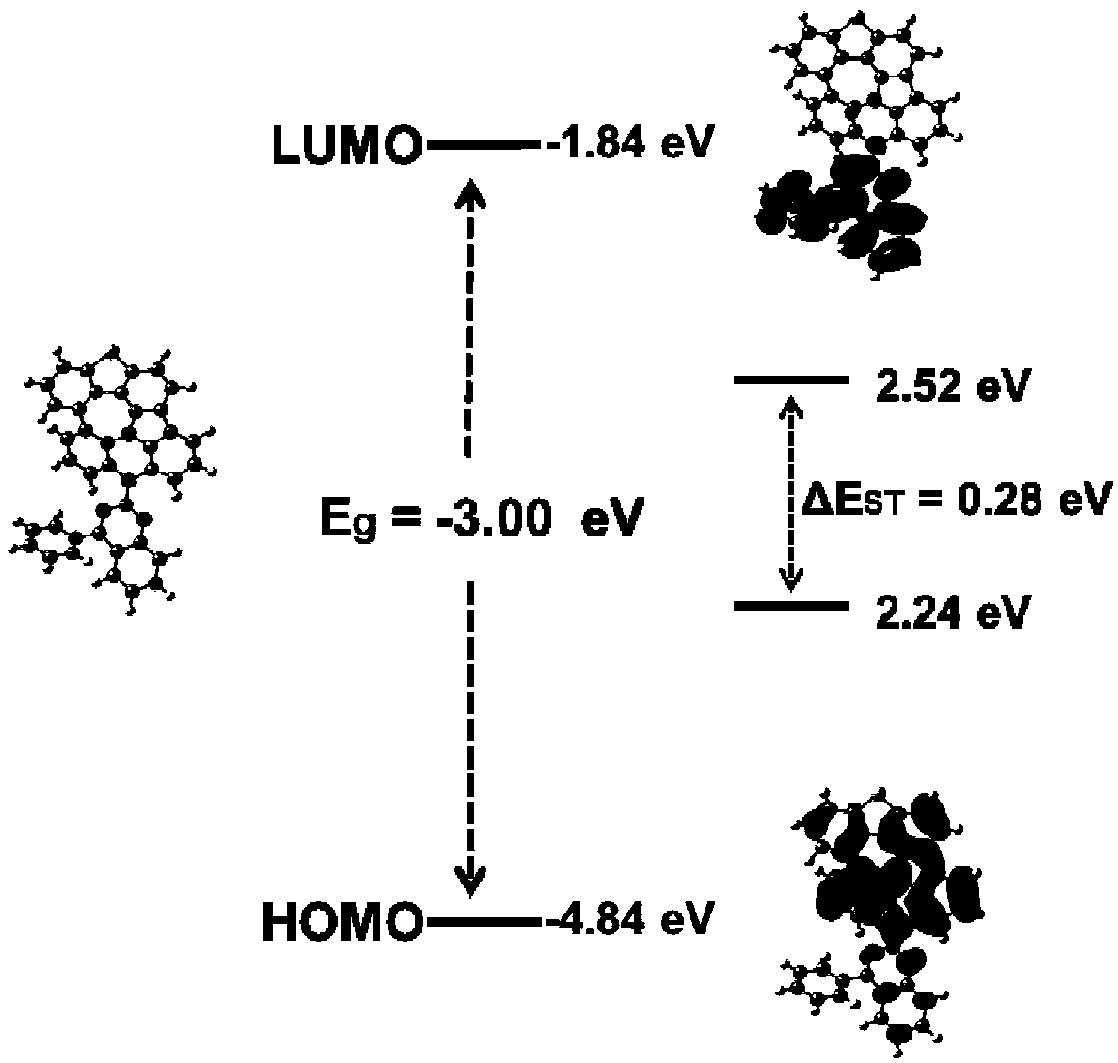
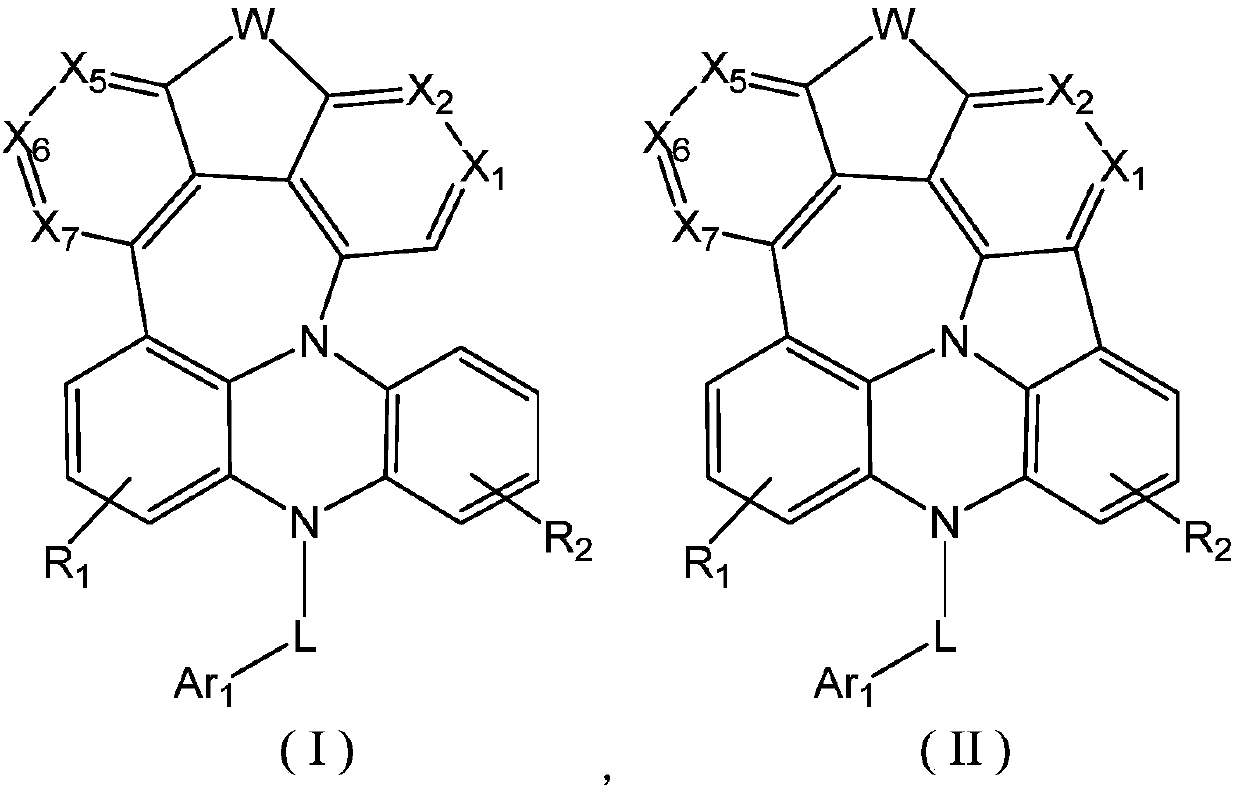
Fused ring compound as well as preparation method and application thereof
A compound and fused ring technology, applied in the field of fused ring compounds and their preparation, can solve the problems of unbalanced charge transport of host materials, unsatisfactory light-emitting area, and inefficient transfer of host material energy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The present embodiment provides a fused ring compound having the structure shown in the following formula D-3:
[0067]
[0068] The synthetic route of the fused ring compound represented by formula D-3 is as follows:
[0069]
[0070] The preparation method of the fused ring compound represented by formula D-3 specifically comprises the following steps:
[0071] (1) Synthesis of intermediate 3-1
[0072] Under nitrogen protection, in the 500mL three-necked flask, add the compound (50mmol) shown in 12.8g formula (C-1), 8.8g 3-chloro-2-fluoronitrobenzene (50mmol) (formula (E-1) The compound shown), 19.5g of cesium carbonate (60mmol), 200mL of dimethyl sulfoxide, reacted for 15 hours, extracted with toluene, evaporated to remove the solvent, passed through a silica gel column to obtain 14.8g of solid intermediate 3-1 (yield 72%) ;
[0073] (2) Synthesis of intermediate 4-1
[0074] Under nitrogen protection, into a 500mL three-necked flask, add 12.4g intermediat...
Embodiment 2
[0081] The present embodiment provides a fused ring compound having the structure shown in the following formula D-4:
[0082]
[0083] The synthetic route of the fused ring compound represented by formula D-4 is as follows:
[0084]
[0085] The preparation method of the fused ring compound represented by formula D-4 specifically comprises the following steps:
[0086] Using the compound represented by formula (C-2) and the compound represented by formula (E-1) as raw materials, according to the synthesis method provided in Example 1, the fused ring compound represented by formula (D-4) was obtained.
[0087] Elemental analysis: (C43H24N6) Theoretical value: C, 82.67; H, 3.87; N, 13.45; Found value: C, 82.61; H, 3.83; N, 13.49, HRMS(ESI) m / z(M+): Theoretical value: 624.21; Found: 624.19.
Embodiment 3
[0089] The present embodiment provides a fused ring compound having the structure shown in the following formula D-1:
[0090]
[0091] The synthetic route of the fused ring compound represented by formula D-1 is as follows:
[0092]
[0093] (1) Synthesis of Intermediate 1-1
[0094] Under nitrogen protection, weigh 8.7g of compound represented by formula (A-1) (30mmol), 6.7g of 1-bromo-2-nitrobenzene (33mmol), 0.2g of palladium acetate (1.0mmol), 0.66g of tri-tertiary Butylphosphine (3.5mmol), 9.3g of sodium tert-butoxide, 1000mL of toluene, reacted at 110°C for 12 hours, cooled to room temperature, extracted with chloroform, evaporated to remove the solvent, and passed through a silica gel column to obtain 10.5g of solid intermediate 1-1 ( Yield 85%);
[0095] (2) Synthetic intermediate 2-1
[0096] Under nitrogen protection, add 8.2g intermediate 1-1 (20mmol), 21.6g stannous chloride dihydrate (96mmol), 17mL hydrochloric acid, 250mL ethanol, react at 60 degrees Ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com