Method for electrochemically coordinating persulfate to remove organic pollutants in wastewater
A technology for organic pollutants and persulfate, applied in water pollutants, chemical instruments and methods, water/sewage treatment, etc., can solve the problems of long-term supply, reduction of cathode electrode current density, low oxidant use efficiency, etc. To achieve the effect of short response time, low consumption and low current
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
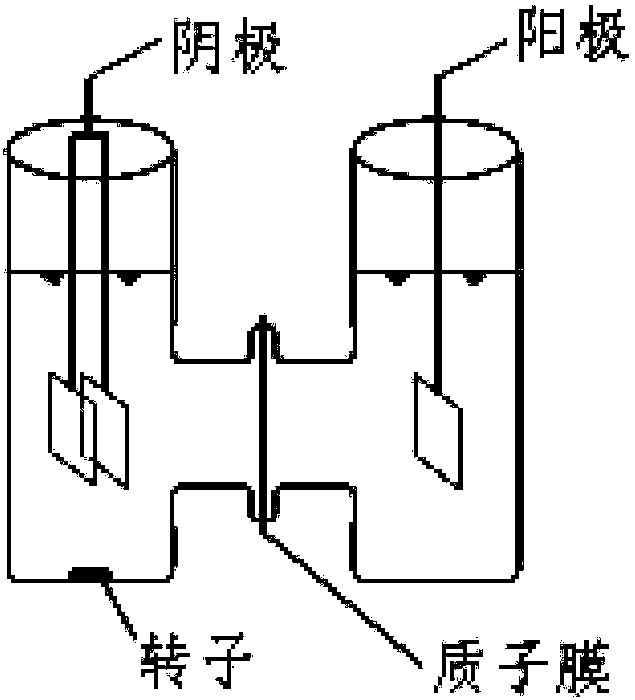
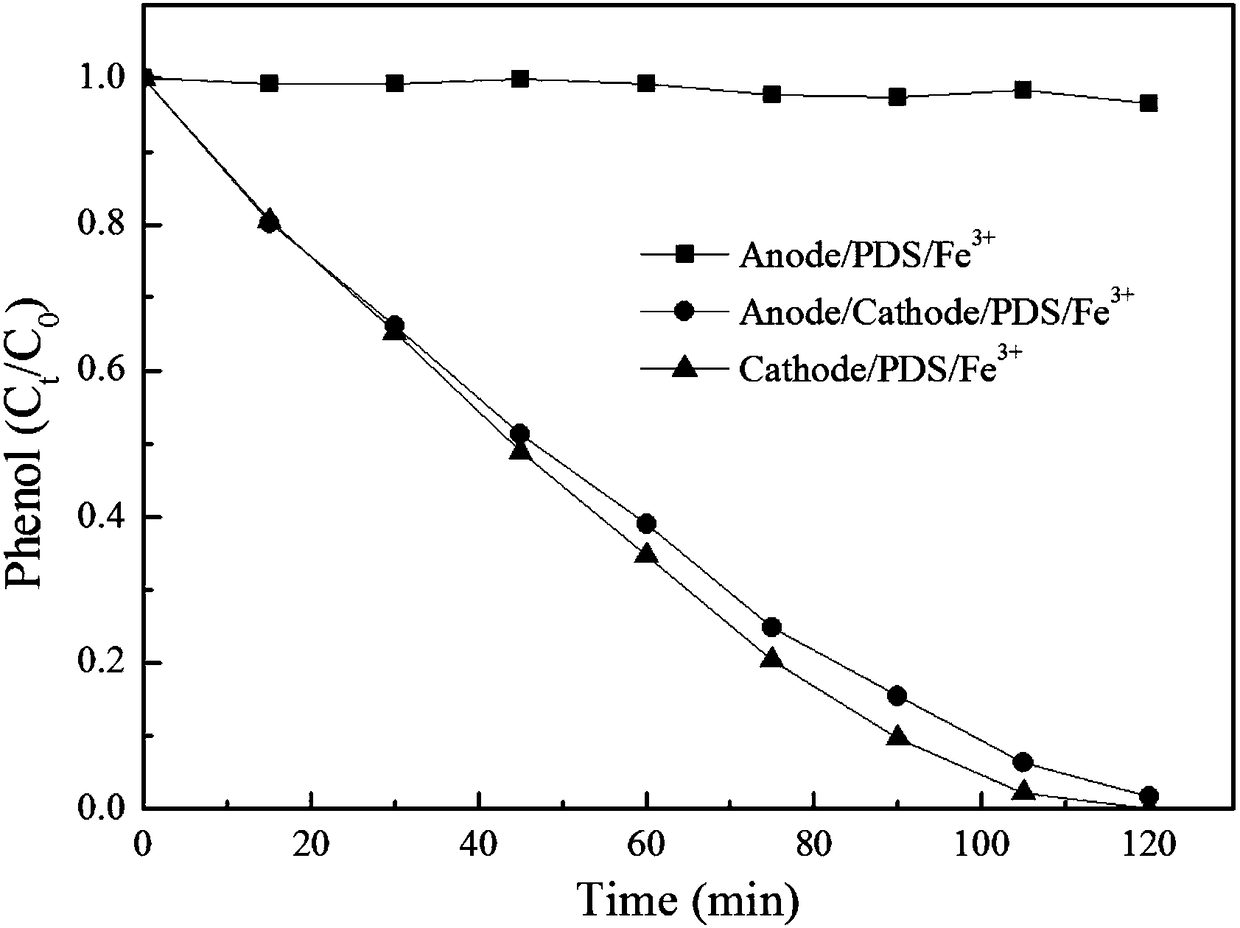
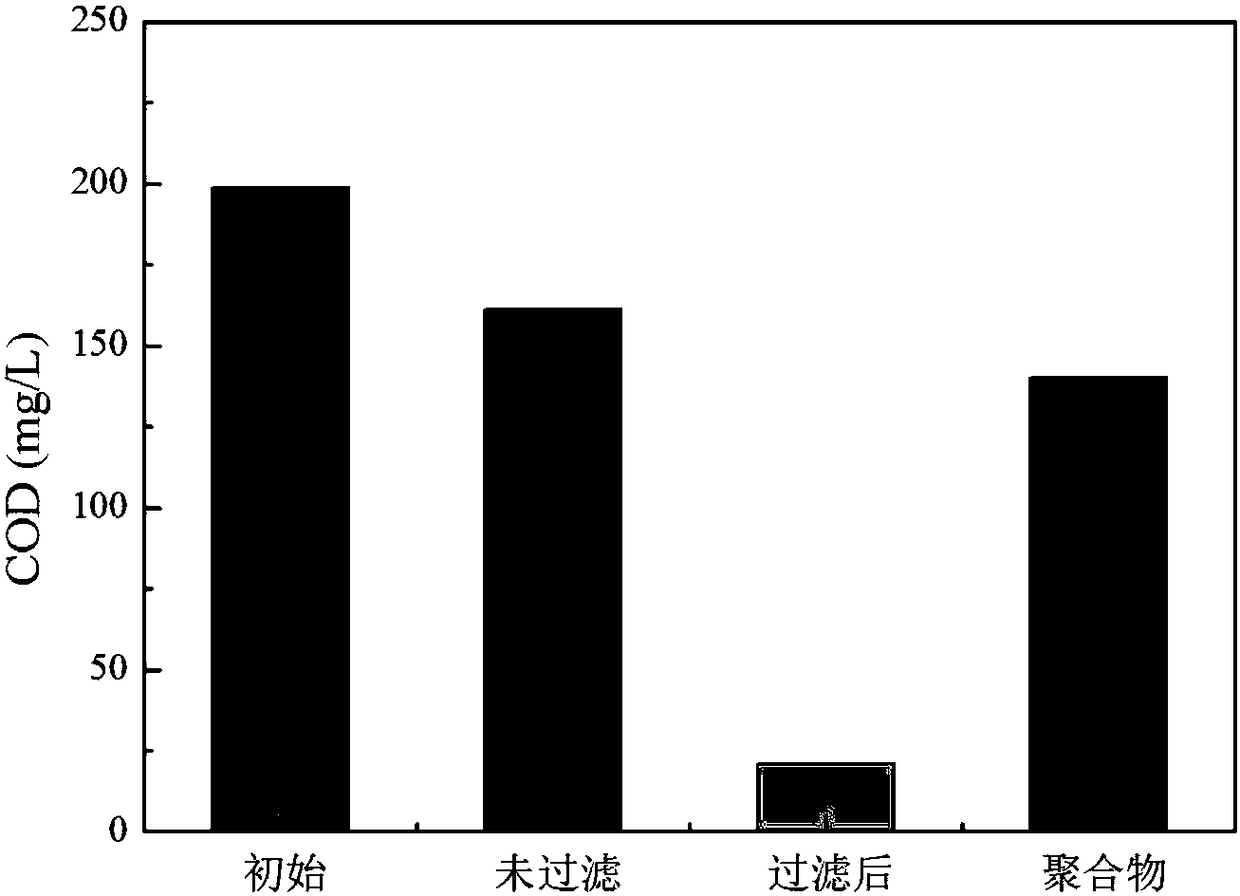
[0045] Contrasting (1) anode / PDS / Fe in this embodiment 3+ , (2) anode / cathode / PDS / Fe 3+ and (3) Cathode / PDS / Fe 3+ The removal effect of phenol in aqueous solution. The results showed that after 2 hours of reaction, the phenol in (1) did not polymerize and was almost not removed; while (2) and (3) had basically the same effect, and both phenol polymerized to form a black solid precipitate. After solid-liquid separation, almost Phenol can be completely removed. This example demonstrates that the anode is essentially ineffective under these conditions and it is primarily the cathode that does the work. The detailed operating conditions are as follows:
[0046] Operating conditions:
[0047] Anode: a 2cm*2cm platinum plate electrode
[0048] Cathode: two 2cm*2cm platinum plate electrodes connected in parallel
[0049] Phenol solution concentration: 1mmol / L
[0050] Phenol solution volume: 100mL
[0051] Sulfate: 50mmol / L sodium sulfate
[0052] Anolyte volume: 100mL
[...
Embodiment 2
[0060] In this example, (1) cathode, (2) cathode / PDS, (3) cathode / Fe 3+ 、 (4)PDS / Fe 3+ And (3) cathode / PDS / Fe in embodiment 1 3+ The removal effect of phenol in aqueous solution. The results show that (1) (2) (3) (4) phenol does not polymerize within 2 hours and cannot be removed substantially. In Example 1 (3) cathode / PDS / Fe 3+ The polymerization of phenol produces a black solid precipitate, which can be almost completely removed after solid-liquid separation. This example illustrates that in Example 1 (3) cathode / PDS / Fe 3+ What works in the system is the whole system, and the medicines added are indispensable. The detailed operating conditions are as follows:
[0061] (1) Separate cathode
[0062] Anode: a 2cm*2cm platinum plate electrode
[0063] Cathode: two 2cm*2cm platinum plate electrodes connected in parallel
[0064] Phenol solution concentration: 1mmol / L
[0065] Phenol solution volume: 100mL
[0066] Sulfate: 50mmol / L sodium sulfate
[0067] Anolyte volum...
Embodiment 3
[0105] In this example, the influence of different amounts of PDS on the removal of phenol in the aqueous solution is compared. The dosage of PDS is respectively 5, 7.5, 10, 15, 20mmol / L, and all the other reaction conditions are the same as those in Example 1 (3) cathode / PDS / Fe 3+ unanimous. The results showed that the more PDS was added, the more phenol was polymerized, the better the removal effect, and the reduction of PDS was basically the same (5 mmol / L within 2 hours), indicating that there was no need to add too much PDS during the reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com