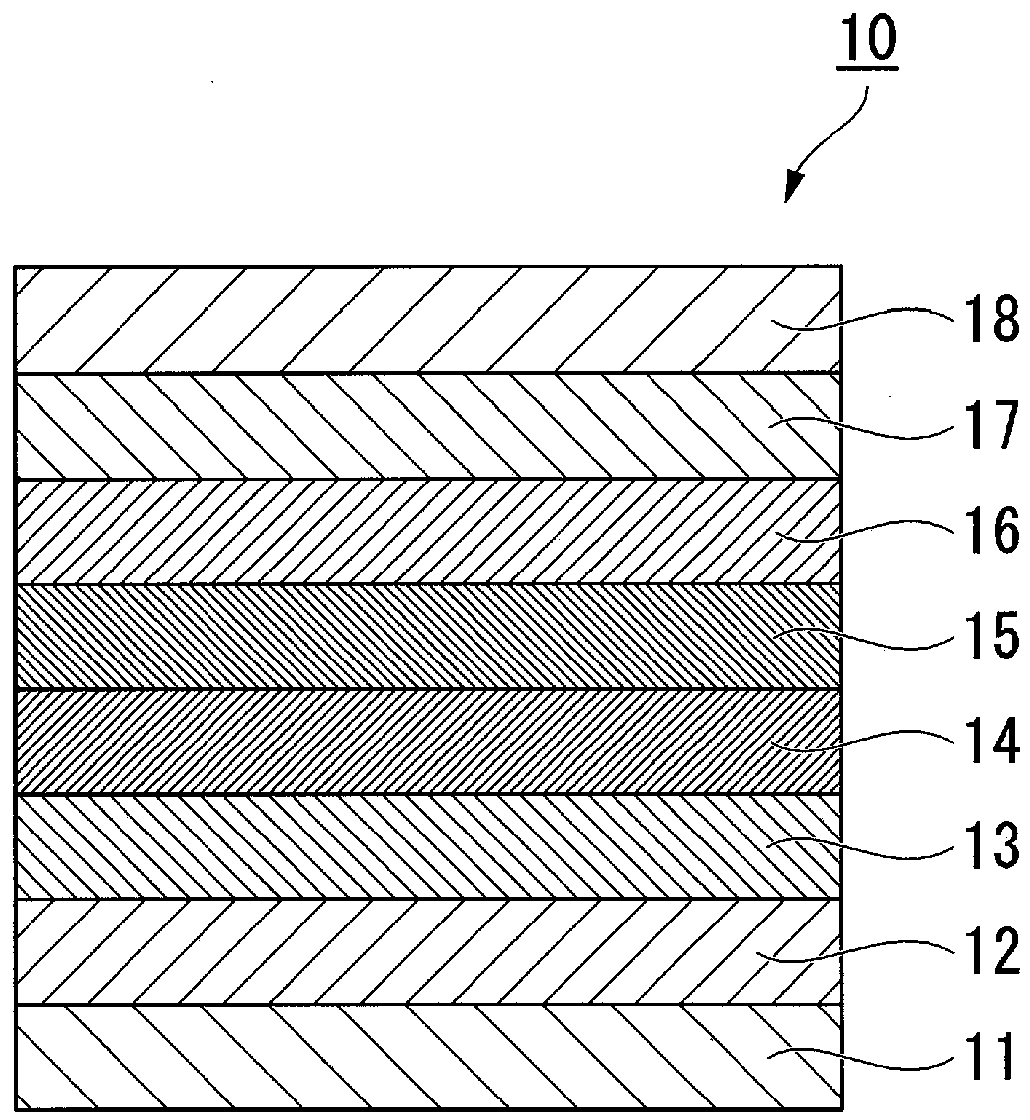
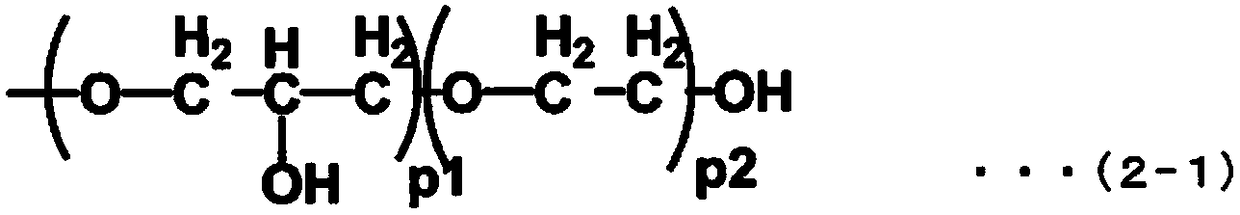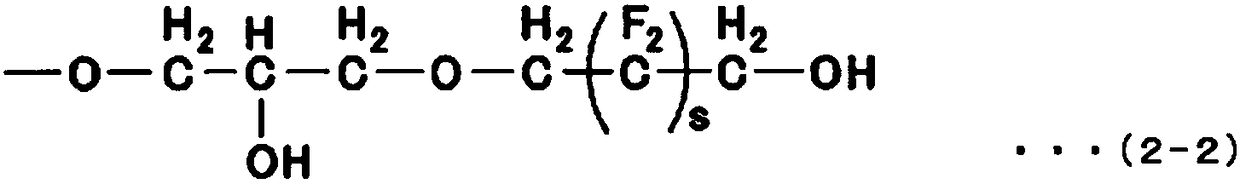Fluorine-containing ether compound, lubricating agent for magnetic recording media, and magnetic recording medium
A magnetic recording medium and compound technology, applied in magnetic recording, magnetic recording layer, data recording, etc., can solve the problems of insufficient durability of magnetic recording media, and achieve excellent reliability and durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0246] The compound represented by the above formula (A) was produced by the method shown below.
[0247] Under a nitrogen atmosphere, add HOCH to a 100 mL eggplant flask 2 CF 2 O(CF 2 CF 2 O) q (CF 2 O) r CF 2 CH 2 25.4 g of a compound represented by OH (q in the formula is 6, and r is 6.) (number average molecular weight: 1270, molecular weight distribution: 1.1), 1.50 g of glycidyl phenyl ether represented by the following formula (7), and t-BuOH 10 mL, stirred at room temperature until homogeneous. 0.900 g of t-BuOK was further added to this homogeneous liquid, and it stirred and reacted at 70 degreeC for 8 hours.
[0248] The obtained reaction product was cooled to 25° C., neutralized with 0.5 mol / L hydrochloric acid, extracted with Bartrel XF (hereinafter referred to as Bartrel XF) manufactured by Mitsui Dupont Flow Chemical Co., Ltd., the organic layer was washed with water, passed through anhydrous sodium sulfate Dehydration was performed. After separating the...
Embodiment 2
[0260] Except having used 1.80 g of the compound represented by following formula (10) instead of the compound represented by formula (7), it carried out similarly to Example 1, and obtained 4.85 g of compounds (B).
[0261] Carry out the compound (B) obtained 1 The structure was identified by H-NMR measurement from the following results.
[0262] compound (B); 1 H-NMR (C 6 f 6 / CD 3 COCD 3 =4 / 1(V / V));
[0263] δ[ppm]3.45~3.60(3H), 3.60~4.00(14H), 3.95~4.15(4H), 6.75~6.85(2H), 7.10~7.20(2H)
[0264]
Embodiment 3
[0266] Except having used 2.00 g of the compound represented by following formula (11) instead of the compound represented by formula (7), it carried out similarly to Example 1, and obtained 4.80 g of compounds (C).
[0267] In addition, a compound represented by the following formula (11) is synthesized from naphthol and epichlorohydrin.
[0268] Carry out the obtained compound (C) 1 The structure was identified by H-NMR measurement from the following results.
[0269] Compound (C); 1 H-NMR (C 6 f 6 / CD 3 COCD 3 =4 / 1(V / V));
[0270] δ[ppm]3.40~3.55(3H), 3.60~3.90(11H), 4.00~4.15(4H), 7.00~7.70(7H)
[0271]
PUM
| Property | Measurement | Unit |
|---|---|---|
| coating thickness | aaaaa | aaaaa |
| coating thickness | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com