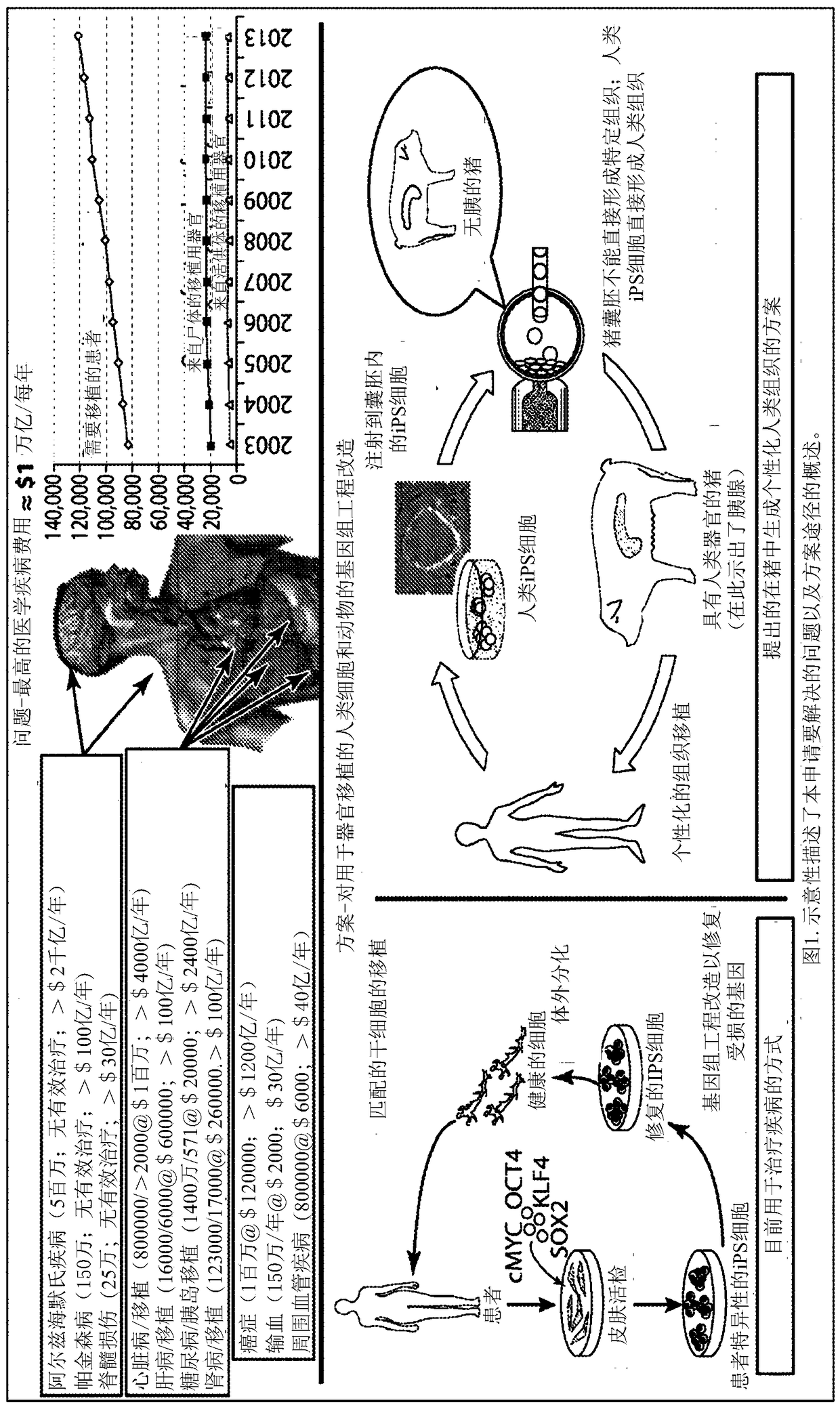
Compositions and methods for chimeric embryo-assisted organ production
A technology of organs and embryos, applied in the field of chimeric embryo-assisted organ preparation compositions and methods, capable of solving problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0350] Example 1, see Figures 4A-4D , describe experiments that successfully attempted to knock out two genes at once using HDR editing and, further, were able to select cells that were homozygous for the double gene knockout or heterozygous for each knockout. Cells are treated to introduce first and second targeting endonucleases directed to a first gene target (recombination-activated gene 2, RAG2) and a second gene target (interleukin receptor 2, gamma, IL2Rg or ILR2 gamma), respectively Enzymes (each a TALEN pair). TALENs must be designed to target a predetermined site and obtain sufficient quantities. Cell processing time is less than 5 minutes. Electroporation is used, but many other suitable methods of protein or DNA introduction are also described herein. The cells are then cultured so that they form a single colony each originating from one treated cell. Cells from different colonies were tested after 3 or 11 days. The knockout rate of RAG2 was approximately 6-f...
Embodiment 2
[0351] Example 2, see Figures 5A-5D , describing multiplex HDR editing experiments with the same target but different genes. The first gene target is the adenomatous polyposis coli gene (APC). The second gene target is p53 (TP53 gene). Cells homozygous for the double knockout and cells heterozygous for the double knockout were detected and isolated.
Embodiment 3
[0352] Example 3, see Figures 6-9, describes multiplex HDR editing to knock out 2-5 genes. There were three experiments, and the number of colonies used to test genotypes in each experiment ranged from 72 to 192. Cells were treated for different combinations, multiplexing of genes APC, p53, RAG2, low density lipoprotein receptor (LDLR), IL2Rg, kisspeptin receptor (KISSR or GPR54) and eukaryotic translation initiation factor 4GI (EIF4GI) knockout. The gene LDLR has always been less susceptible to modification than other genes. As can be seen from the results, with TALEN-specific homology-mediated repair (HDR), multiple alleles can be destroyed simultaneously. Five TALEN pairs were co-transfected in three combinations (Table A), where each of the five TALEN pairs resulted in more than 20% HDR / site and its cognate HDR template. A fraction of colonies from each replicate were positive for HDR events in at least four genes, and two colonies from repeat-A had HDR events in five g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com