Content determination method of magnoflorine in dichocarpum sutchuenense medicinal material
A magnolidine and determination method technology, which is applied in the directions of measuring device, material separation, analysis of materials, etc., can solve problems such as difficulty in controlling the quality of medicinal materials and their formulations, restricting the development and utilization of herringbone fruit, and no quantitative detection of medicinal effect components, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
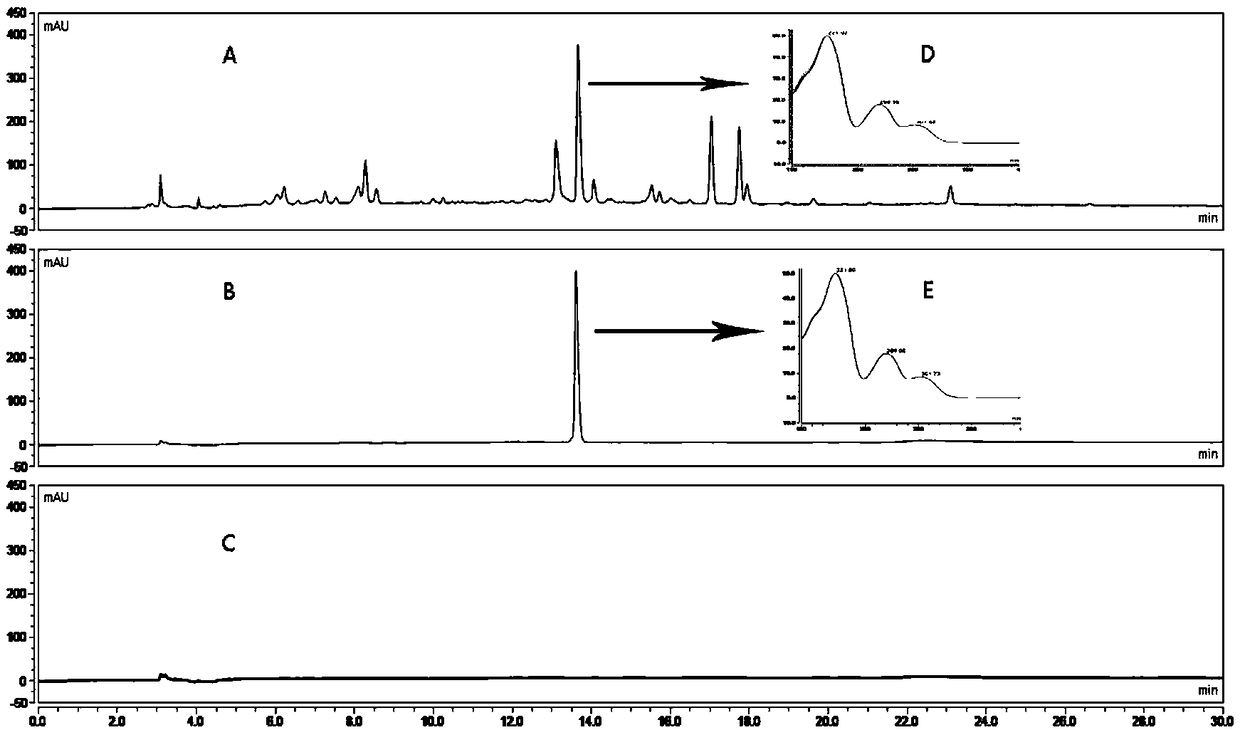
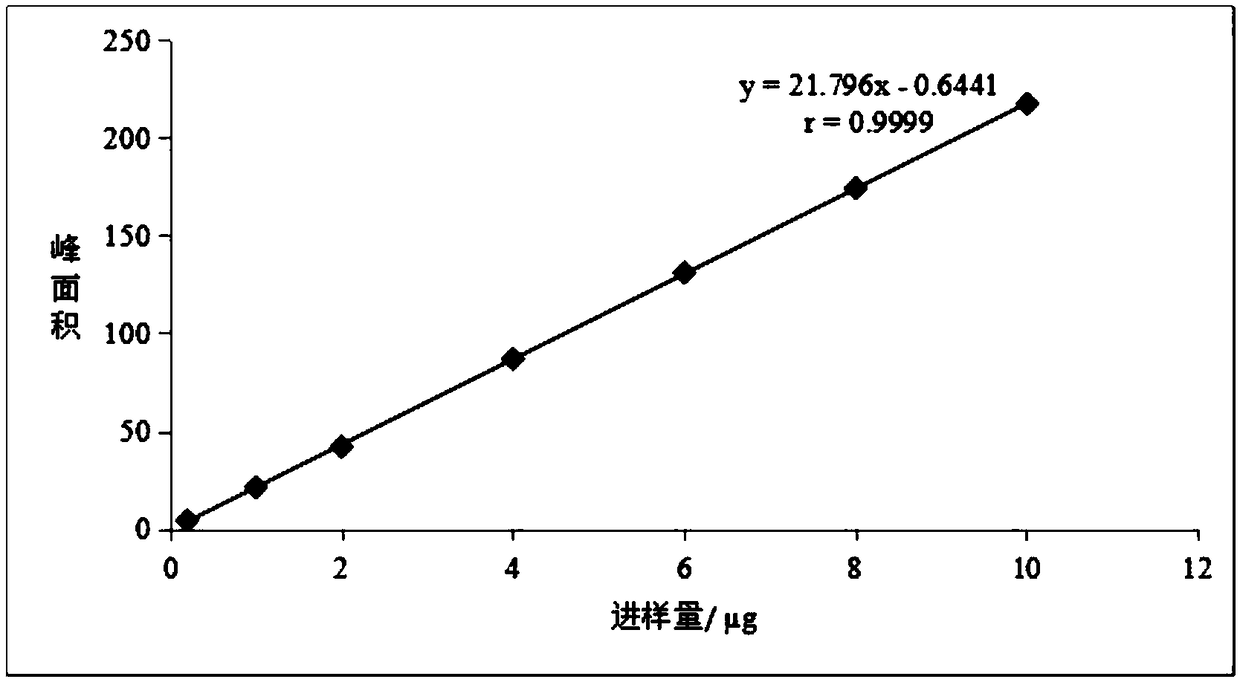
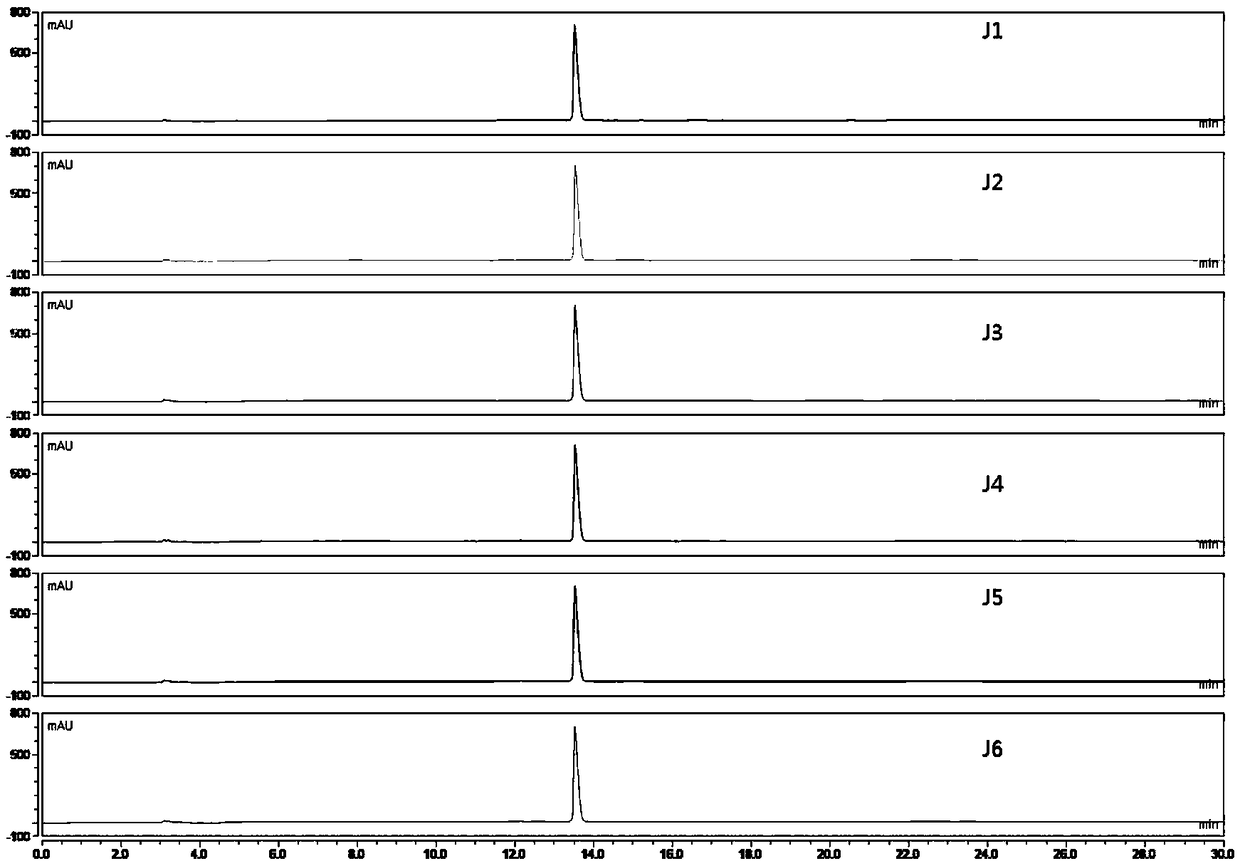
[0075] A method for assaying the content of magnolanine in the herb fruit medicinal material, the method for assaying the content of magnolanine is:
[0076] Chromatographic conditions: Pntulips BP-C 18 Column (4.6mm×250mm, 2.5μm); mobile phase: mobile phase A is acetonitrile, mobile phase B is 0.1% phosphoric acid-0.1% triethylamine aqueous solution, gradient elution program: 0-10min, 5%-20% A, 10~30min, 20%~40%A; detection wavelength: 270nm; column temperature: 25°C; flow rate: 1mL min -1 ;Injection volume: 10μL;
[0077] Preparation of reference substance solution: Accurately weigh an appropriate amount of magnolialine reference substance, put it in a volumetric flask, add 75% methanol solution to make 0.40032 mg·mL -1 The reference substance solution;
[0078] Preparation of the test solution: take 1.0 g of herb powder, accurately weigh it, place it in a stoppered Erlenmeyer flask, add 25 mL of 75% methanol solution accurately, weigh it, and extract it ultrasonically fo...
Embodiment 2
[0081] A method for assaying the content of magnolanine in the herb fruit medicinal material, the method for assaying the content of magnolanine is:
[0082] Chromatographic conditions: Pntulips BP-C 18 Column (4.6mm×250mm, 2.5μm); mobile phase: mobile phase A is acetonitrile, mobile phase B is 0.1% phosphoric acid-0.1% triethylamine aqueous solution, gradient elution program: 0-10min, 5%-20% A, 10~30min, 20%~40%A; detection wavelength: 270nm; column temperature: 25°C; flow rate 0.8mL min -1 ;Injection volume 10μL;
[0083] Preparation of reference substance solution: Accurately weigh magnolialine reference substance, put it in a volumetric flask, add 80% methanol solution to make 0.50032 mg·mL -1 The reference substance solution;
[0084] Preparation of the test solution: take 1.0 g of herb powder, accurately weigh it, place it in a stoppered Erlenmeyer flask, add 20 mL of 50% methanol solution accurately, weigh it, and extract it ultrasonically for 30 min at a power of 10...
Embodiment 3
[0087] A method for assaying the content of magnolanine in the herb fruit medicinal material, the method for assaying the content of magnolanine is:
[0088] Chromatographic conditions: Pntulips BP-C 18 Column (4.6mm×250mm, 2.5μm); mobile phase: mobile phase A is acetonitrile, mobile phase B is 0.1% phosphoric acid-0.1% triethylamine aqueous solution, gradient elution program: 0-10min, 5%-20% A, 10~30min, 20%~40%A; detection wavelength: 280nm; column temperature: 30°C; flow rate: 1.2mL min -1 ;Injection volume 10μL;
[0089] Preparation of reference substance solution: Accurately weigh an appropriate amount of magnolialine reference substance, put it in a volumetric flask, add 75% methanol solution to make 0.40032 mg·mL-1 The reference substance solution;
[0090] Preparation of the test solution: Take about 1.2g of the herb powder, accurately weigh it, put it in a stoppered Erlenmeyer flask, add 50mL of 90% methanol solution accurately, weigh it, and extract it by ultrasoni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com