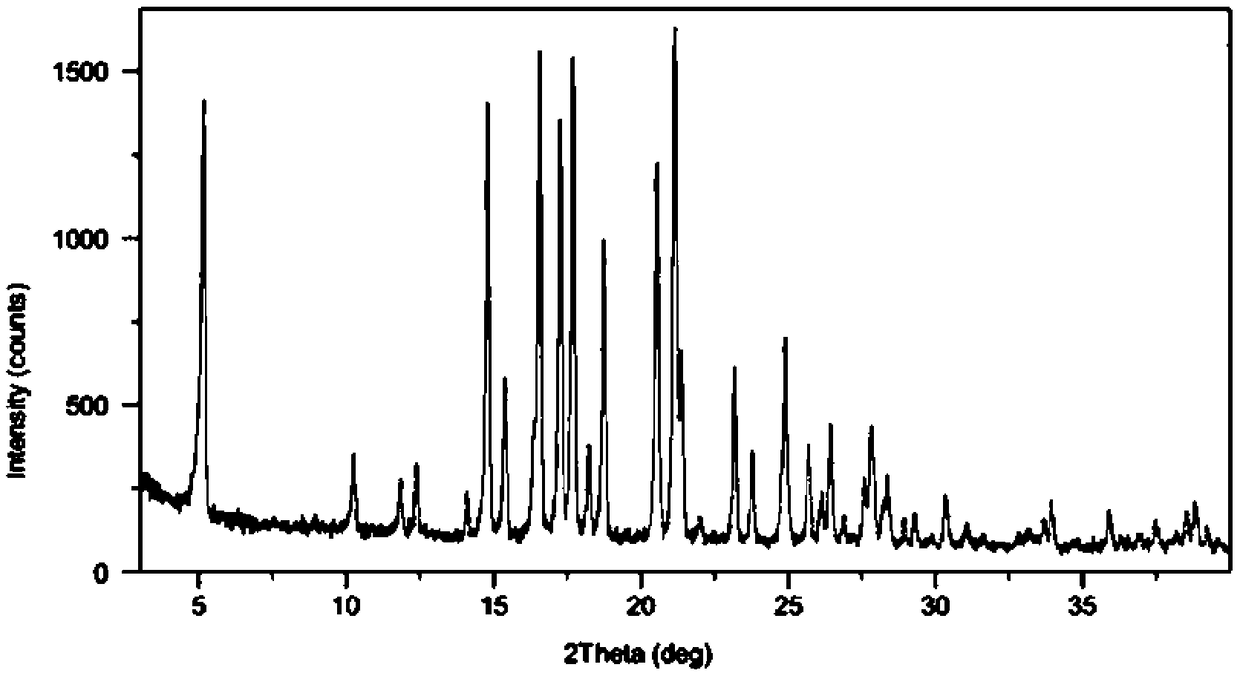
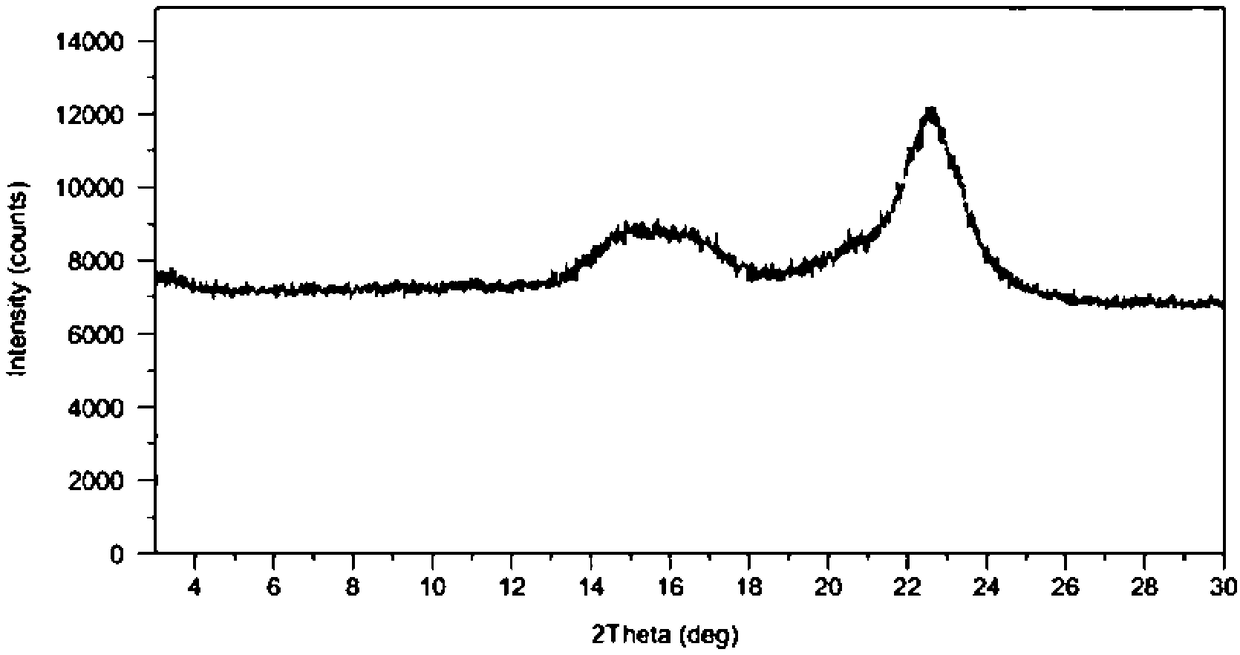
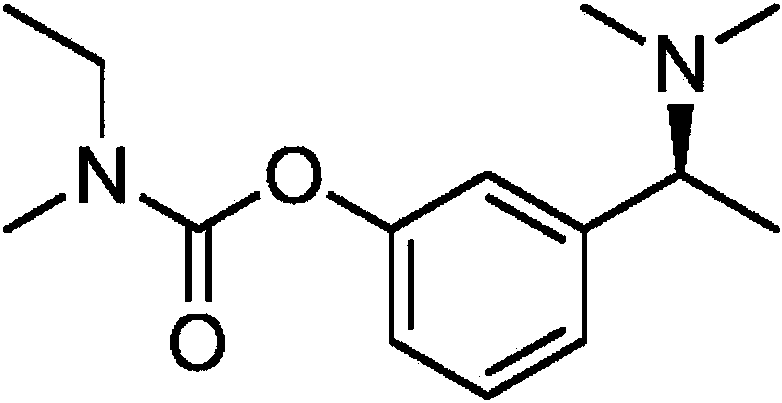
Rivastigmine transdermal patch and preparation method thereof
A technology of transdermal patch and spray drying method, which is applied in the directions of osmotic delivery, pharmaceutical formulations, medical preparations of inactive ingredients, etc. Skin release rate and other issues, to achieve the effect of less drug irritation, simple preparation process, and improved transdermal release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A preparation method of rivastigmine transdermal patch, comprising:
[0036] (1) The preparation method of the powder of rivastigmine or its salt, it is by rivastigmine or its salt, and phospholipid and water-soluble adjuvant are dispersed in dehydrated alcohol, and described phospholipid is selected from soybean lecithin; Sexual excipients are selected from polyvinylpyrrolidone, which is prepared by spray drying method. The weight ratio of rivastigmine to phospholipids and water-soluble excipients is 70:25:5. The spray drying process is nitrogen flow pressure: 600L / h; Temperature: 90°C; Outlet temperature: 43°C; Feed rate: 5mL / min; Needle pass frequency: 15 seconds / time;
[0037] (2) The preparation method of the adhesive layer of transdermal patch: the adhesive layer is liquid paraffin (mass ratio 80%) and styrene-isoprene-styrene block copolymer (mass ratio 20%), in acetic acid Disperse and mix in an ethyl ester solvent, prepare an adhesive layer according to the th...
Embodiment 2
[0040] A preparation method of rivastigmine transdermal patch, comprising:
[0041] (1) The preparation method of the powder of rivastigmine or its salt, it is by rivastigmine or its salt, and phospholipid and water-soluble adjuvant are dispersed in dehydrated alcohol, and described phospholipid is selected from soybean lecithin; Sexual excipients are selected from polyethylene glycol 6000, which is prepared by spray drying method. The weight ratio of rivastigmine to phospholipids and water-soluble excipients is 70:25:5. The spray drying process is nitrogen flow pressure: 600L / h; Inlet air temperature: 90°C; Outlet air temperature: 43°C; Feed rate: 5mL / min; Needle pass frequency: 15 seconds / time;
[0042](2) The preparation method of the adhesive layer of transdermal patch: the adhesive layer is liquid paraffin (mass ratio 80%) and styrene-isoprene-styrene block copolymer (mass ratio 20%), in acetic acid Disperse and mix in an ethyl ester solvent, prepare an adhesive layer ac...
Embodiment 3
[0045] A preparation method of rivastigmine transdermal patch, comprising:
[0046] (1) The preparation method of the powder of rivastigmine or its salt, it is by rivastigmine or its salt, and phospholipid and water-soluble adjuvant are dispersed in dehydrated alcohol, and described phospholipid is selected from soybean lecithin; Sexual excipients are selected from polyvinylpyrrolidone, which is prepared by spray drying method. The weight ratio of rivastigmine to phospholipids and water-soluble excipients is 70:25:5. The spray drying process is nitrogen flow pressure: 600L / h; Temperature: 90°C; Outlet temperature: 43°C; Feed rate: 5mL / min; Needle pass frequency: 15 seconds / time;
[0047] (2) The preparation method of the adhesive layer of transdermal patch: adhesive layer is liquid paraffin (mass ratio 70%) and styrene-isoprene-styrene block copolymer (mass ratio 30%), in acetic acid Disperse and mix in an ethyl ester solvent, prepare an adhesive layer according to the thickn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com