Method for recycling fluorine and lithium from waste electrolyte of lithium battery
A technology for waste electrolyte and lithium battery, applied in battery recycling, waste collector recycling, secondary battery and other directions, can solve the problems of easily polluted air environment, polluted groundwater, polluted the environment, etc., to reduce pollution, save costs, treat low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
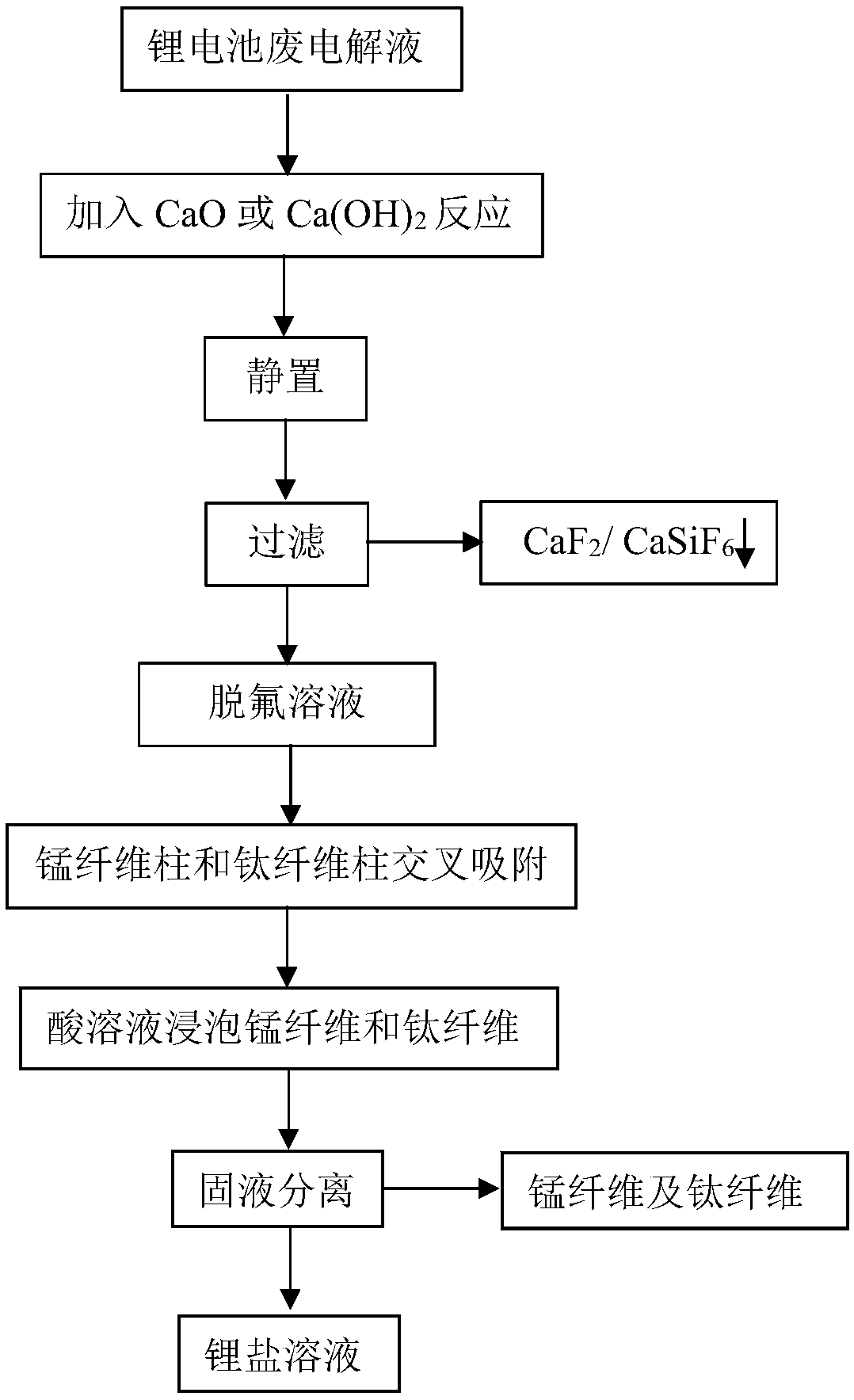
[0029] (1) Add CaO or Ca(OH) to lithium battery waste electrolyte 2 Reaction for 3 hours, Ca 2+ React with fluoride ions in the electrolyte to generate CaF 2 Precipitation, static layering, and then solid-liquid separation to obtain CaF 2 and defluorinated solution. CaO or Ca(OH) 2 The amount of addition is calculated according to the content of fluoride ions in the electrolyte, and the amount of addition is sufficient to meet the complete reaction of fluoride ions.
[0030] (2) Pass the defluorination solution into an adsorption device containing manganese fibers and titanium fibers for adsorption treatment, and the manganese fibers and titanium fibers enrich lithium ions in the defluorination solution. The adsorption device is composed of manganese fiber adsorption columns and titanium fiber adsorption columns alternately connected in series, with 5 manganese fiber adsorption columns and 5 titanium fiber adsorption columns, that is, the adsorption device is equipped with...
Embodiment 2
[0033] (1) Add CaO and SiO to lithium battery waste electrolyte 2 After 10 hours of reaction, Ca 2+ React with fluoride ions in the electrolyte to generate CaF 2 and CaSiF 6 Precipitation, static layering, and then solid-liquid separation to obtain CaF 2 、CaSiF 6 and defluorinated solution. CaO and SiO 2 The amount of addition is calculated according to the content of fluoride ions in the electrolyte, and the amount of addition is sufficient to meet the complete reaction of fluoride ions.
[0034](2) Pass the defluorination solution into an adsorption device containing manganese fibers and titanium fibers for adsorption treatment, and the manganese fibers and titanium fibers enrich lithium ions in the defluorination solution. The adsorption device is composed of manganese fiber adsorption columns and titanium fiber adsorption columns alternately connected in series, with 6 manganese fiber adsorption columns and 6 titanium fiber adsorption columns, that is, the adsorption...
Embodiment 3
[0037] (1) Add Ca(OH) to lithium battery waste electrolyte 2 and SiO 2 Reaction for 8 hours, Ca 2+ React with fluoride ions in the electrolyte to generate CaF 2 and CaSiF 6 Precipitation, static layering, and then solid-liquid separation to obtain CaF 2 、CaSiF 6 and defluorinated solution. Ca(OH) 2 and SiO 2 The amount of addition is calculated according to the content of fluoride ions in the electrolyte, and the amount of addition is sufficient to meet the complete reaction of fluoride ions.
[0038] (2) Pass the defluorination solution into an adsorption device containing manganese fibers and titanium fibers for adsorption treatment, and the manganese fibers and titanium fibers enrich lithium ions in the defluorination solution. The adsorption device is composed of manganese fiber adsorption columns and titanium fiber adsorption columns alternately connected in series, with 7 manganese fiber adsorption columns and 7 titanium fiber adsorption columns, that is, the ads...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com