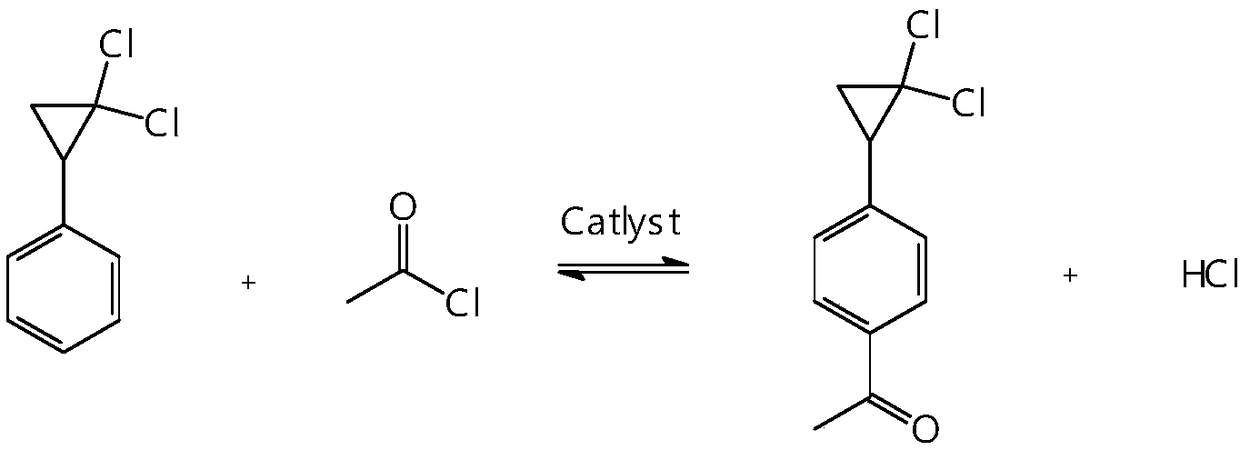
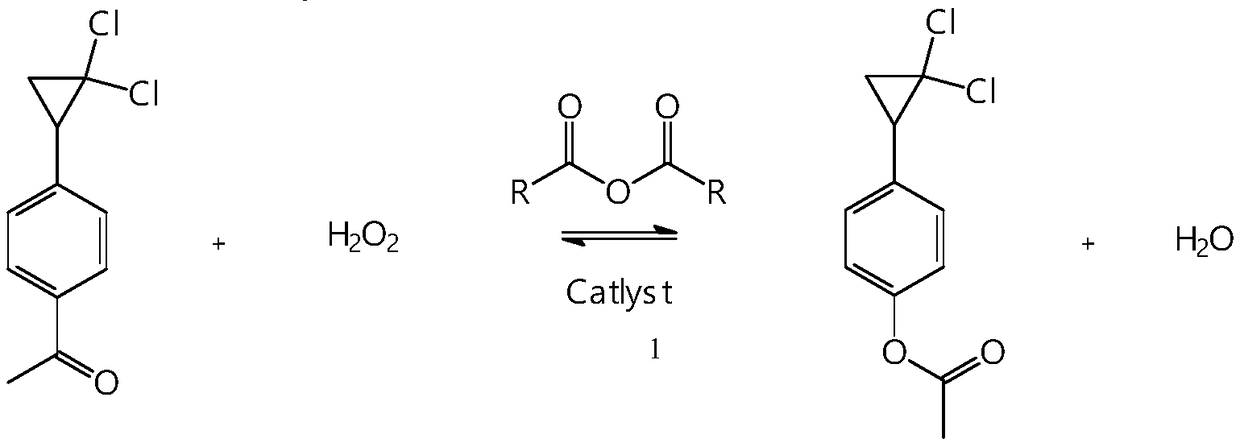
Synthetic method of 4-(2,2-dichloro cyclopropyl)phenol acetate
A technology of dichlorocyclopropylbenzene and dichlorocyclopropyl, which is applied in the field of fine organic chemical synthesis, can solve the problems of cumbersome post-treatment process, poor economy, and increased process cost, so as to improve reaction selectivity and oxidation capacity , Improve the effect of reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] In a dry 500ml four-neck flask equipped with a stirring paddle, a thermometer, and a reflux condenser (the outlet of the condenser is connected to the tail gas absorption system), put into the dried dichloromethane: 250g, anhydrous AlCl 3 : 65g, I 2 : 0.2g. The four-necked flask was cooled externally with a cooling tank refrigerant, and the temperature of the cooling tank refrigerant was set at -20°C. Turn on stirring to lower the temperature, and when the internal temperature reaches -10°C, slowly add 50 g of acetyl chloride (content 98.5%, 0.6274 mol). When the internal temperature reaches -15°C, add 2,2-dichlorocyclopropylbenzene: 79.5 g (content: 98.8%, 0.4200 mol) dropwise into the reaction bottle, and control the dropping time at about 0.5 hours. After the dropwise addition is completed, the internal temperature is -15~-10°C and the reaction is kept for 8 hours. After the heat preservation is completed, 100 ml (temperature: 5-10° C.) of distilled water is added...
Embodiment 2
[0058] In a dry 500ml four-neck flask equipped with a stirring paddle, a thermometer, and a reflux condenser (the outlet of the condenser is connected to the tail gas absorption system), put into the dried dichloromethane: 250g, anhydrous AlCl 3 : 65.7g, ZnI 2 : 0.5g. The four-necked flask was cooled externally with a cooling tank refrigerant, and the temperature of the cooling tank refrigerant was set at -20°C. Turn on stirring to lower the temperature, and when the internal temperature reaches -10°C, slowly add 50 g of acetyl chloride (content 98.5%, 0.6274 mol). When the internal temperature reaches -15°C, add 2,2-dichlorocyclopropylbenzene: 79.4 g (content: 98.8%, 0.4195 mol) dropwise into the reaction bottle, and control the dropping time at about 0.5 hours. After the dropwise addition is completed, the internal temperature is -15~-10°C and the reaction is kept for 8 hours. After the heat preservation is completed, 100 ml (temperature: 5-10° C.) of distilled water is a...
Embodiment 3
[0063] In a dry 500ml four-neck flask equipped with a stirring paddle, a thermometer, and a reflux condenser (the outlet of the condenser is connected to the tail gas absorption system), put into the dried dichloromethane: 250g, anhydrous AlCl 3 : 65g, AlI 3 : 0.5g. The four-necked flask was cooled externally with a cooling tank refrigerant, and the temperature of the cooling tank refrigerant was set at -20°C. Turn on stirring to lower the temperature, and when the internal temperature reaches -10°C, slowly add 50 g of acetyl chloride (content 98.5%, 0.6274 mol). When the internal temperature reaches -15°C, add 2,2-dichlorocyclopropylbenzene: 79.5 g (content: 98.8%, 0.4200 mol) dropwise into the reaction bottle, and control the dropping time at about 0.5 hours. After the dropwise addition is completed, the internal temperature is -15~-10°C and the reaction is kept for 8 hours. After the heat preservation is completed, 100 ml (temperature: 5-10° C.) of distilled water is add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com