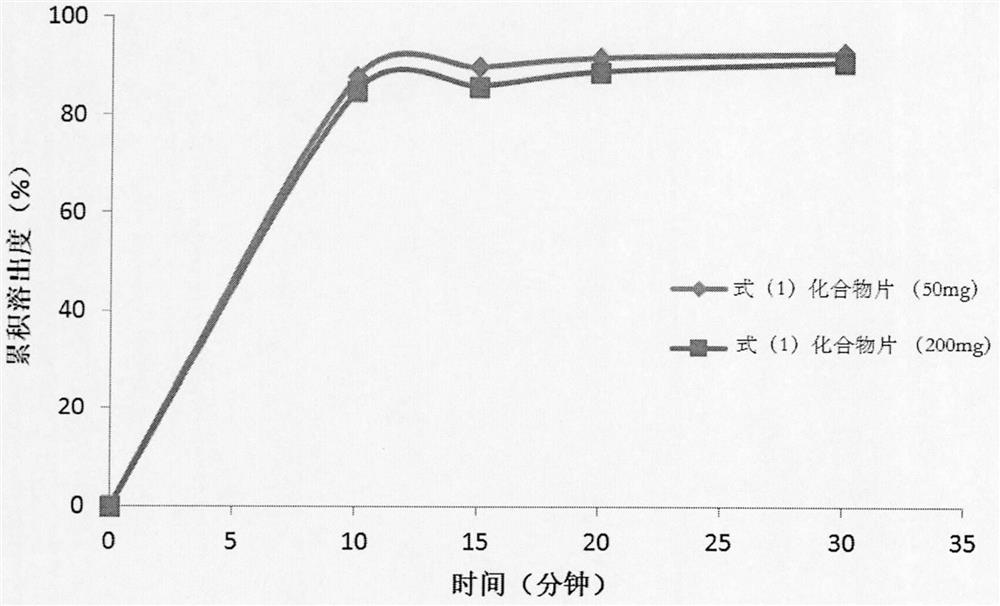
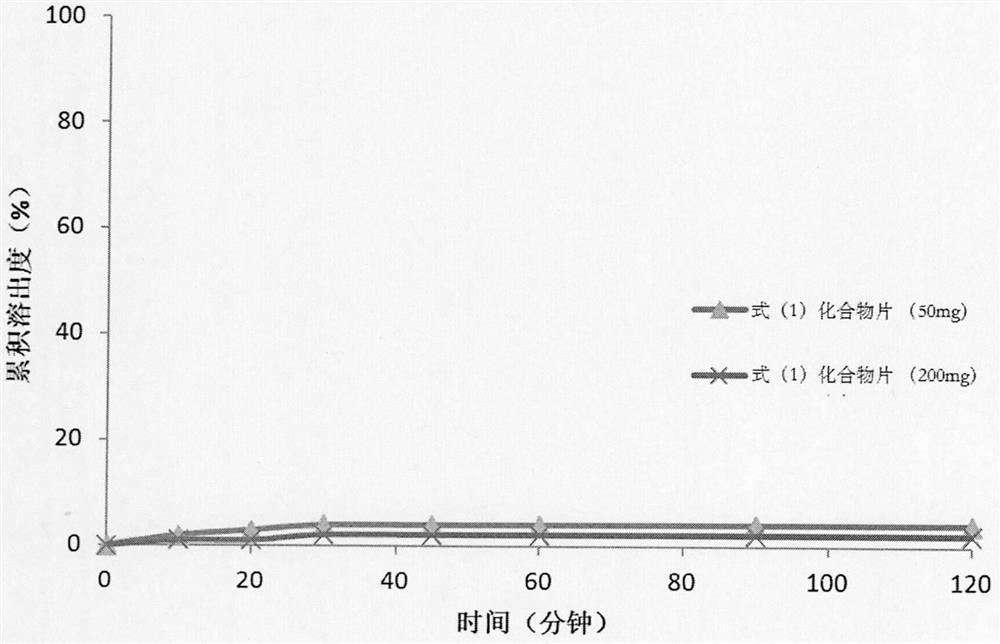
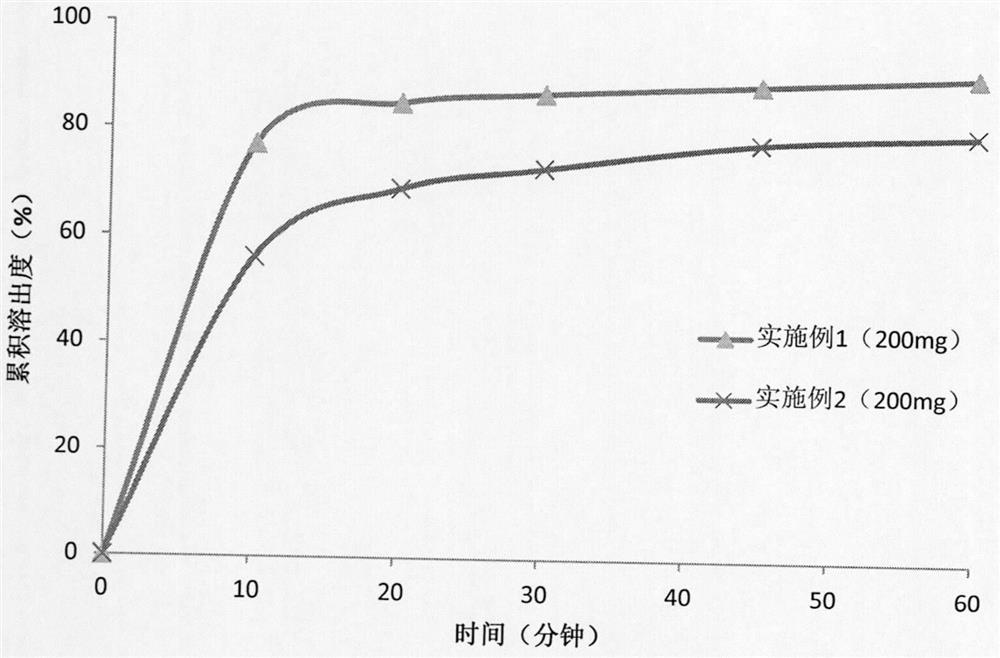
Pharmaceutical preparation composition of pyrrolopyrimidine derivatives as alk/fak/igf1r multi-kinase inhibitors and preparation method thereof
A technology of pharmaceutical preparations and compositions, which is applied in the field of pharmaceutical preparations of pyrrolopyrimidine derivatives, and can solve problems such as degradation impurities, easy oxidation, low bioavailability, easy oxidation instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Formula (I) compound sheet prescription is as follows:
[0021] Name of raw material Dosage (%, w / w) Compound of formula (I) 10 silica 5 Microcrystalline Cellulose PH102 75 Croscarmellose Sodium 3 Sodium dodecyl sulfate 6 Magnesium stearate 1 Coating powder 3
[0022] Formula (I) compound sheet preparation process is as follows:
[0023] 1) Mix 80g of the compound of formula (I) and 40g of silicon dioxide and pass through a 40-mesh sieve. The particle size of the compound of formula (I) is about 7.892 μm in D50 and about 19.172 μm in D90.
[0024] 2) Put the mixture of the sieved formula (I) compound and silicon dioxide with 600g microcrystalline cellulose PH102, 24g croscarmellose sodium, 48g sodium lauryl sulfate, 4g magnesium stearate In the HSD30 hopper mixer (Zhejiang Canaan Pharmaceutical Equipment Co., Ltd.), set the rotation speed at 12 rpm, and mix for 10 minutes.
[0025] 3) Put the material into the...
Embodiment 2
[0030] Formula (I) compound sheet prescription is as follows:
[0031] Name of raw material Dosage (%, w / w) Compound of formula (I) 28.5 silica 5 Microcrystalline Cellulose PH102 60.5 Croscarmellose Sodium 5 Magnesium stearate 1 Coating powder 3
[0032] Formula (I) compound sheet preparation process is as follows:
[0033] 1) Mix 228g of the compound of formula (I) and 40g of silicon dioxide and pass through a 40-mesh sieve. Wherein the particle size of the compound of formula (I) is the same as that of Example 1.
[0034] 2) Place the mixture of the sieved formula (I) compound and silicon dioxide with 484g microcrystalline cellulose PH102, 40g croscarmellose sodium, and 4g magnesium stearate in the HSD30 hopper mixer, set Mix at 12 rpm for 10 minutes.
[0035] 3) Put the material into the hopper of the GL2-25 dry granulator for dry granulation, set the feeding frequency to 6-30Hz, the tableting frequency to 10-30Hz, and ...
Embodiment 3
[0040] Formula (I) compound sheet prescription is as follows:
[0041]
[0042]
[0043] Formula (I) compound sheet preparation process is as follows:
[0044] 1) Weigh 24g of povidone K30 and 24g of poloxamer 188, dissolve in an appropriate amount of purified water to form a binder solution;
[0045] 2) 222.4g of the compound of formula (I), 24g of silicon dioxide, 297.6g of microcrystalline cellulose PH101 and 24g of croscarmellose sodium were placed in a G6 wet mixing granulator (Shenzhen Xinyite Technology Co., Ltd. Company), set the stirring paddle rotating speed 2r / sec, shear velocity 15r / sec, mix 10 minutes;
[0046] 3) Spray into the binder solution for wet granulation, the atomization pressure is about 0.4MPa, and granulate through a 20-mesh sieve;
[0047] 4) Dry the wet granules at 60°C until the water content is less than 2%, and sieve the granules with a 30-mesh sieve;
[0048] 5) Put the granules, 144g of microcrystalline cellulose PH102, and 32g of cros...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com