A kind of synthetic method of ether aminated cyclodextrin derivative
A synthesis method and cyclodextrin technology are applied in the synthesis field of ether-aminated cyclodextrin derivatives, which can solve the problems of not increasing the inclusion ratio of biologically active molecules, not improving biologically active substances, and needing a large amount of biologically active substances, and achieving improved stability. performance, reduce usage, and increase package load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Synthesis of mono-6-deoxy-polyetheramine D400 cyclodextrin (β-CD-6-D400)
[0026] Dissolve β-cyclodextrin (β-CD) (17.0g, 15.0mmol) in 200mL of 1% NaOH solution, and add 15mL of p-toluenesulfonyl chloride (4.0g, 22.5mmol) dropwise at 0°C ) in acetonitrile solution, stirred and reacted at 25°C for 2h, then filtered, adjusted to pH 2 with hydrochloric acid, kept at 4°C for 14h, a large amount of precipitate precipitated out, filtered, and the solid was recrystallized 3 times to obtain a white solid, which was vacuum-dried at 40°C for 5h. Obtain β-CD-6-OTs for future use.
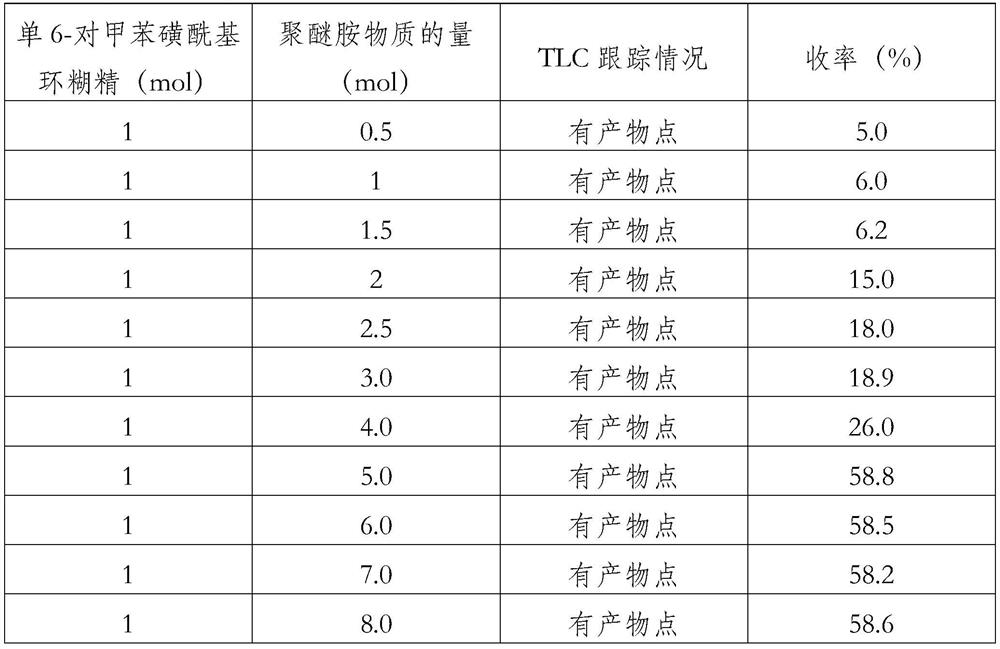
[0027] Mix β-CD-6-OTs (0.50g, 0.39mmol) and polyetheramine D400 (1.2g, 3.00mmol), add 10mL of DMF solvent to dissolve completely, use 1mol / L hydrochloric acid solution to adjust the pH of the system to 5.5, 60 ℃ reaction 24h. The reaction solution was concentrated and dialyzed in water at 40° C. for 2 days using a dialysis bag with a molecular weight cut-off of 1500, then concentrated and separated ...
Embodiment 2
[0034] 1. Synthesis of mono-6-deoxy-polyetheramine D2000 cyclodextrin (β-CD-6-D2000)
[0035] Mix β-CD-6-OTs (0.50g, 0.39mmol) and polyetheramine D2000 (1.6g, 0.80mmol), add 10mL of DMF solvent to dissolve completely, and use 1mol / L hydrochloric acid solution to adjust the pH of the system to 6.5, 100 ℃ reaction 2h. The reaction solution was concentrated and dialyzed in water at 40° C. for 2 days using a dialysis bag with a molecular weight cut off of 3500. Then concentrate and separate with a silica gel column to obtain β-CD-6-D2000 white powder. The elution used is gradient elution, and the gradient elution agent is composed of n-butanol, ethanol, water, and ammonia water in a certain proportion, and the proportion is 6:2:2:1 in terms of volume ratio. β-CD-6-D2000, 1 H NMR (400MHz, DMSO-d 6 ) δ = 5.60-5.75 (m, 14H), 4.82 (s, 7H), 4.36 (s, 6H), 3.00-3.40 (m, 37H), 1.54-0.79 (m, 6H). MALDI-TOF-MS: calculated m / z [M+H 2 O] 3134.6; measured value, 3134.7 [M+H 2 O].
[00...
Embodiment 3
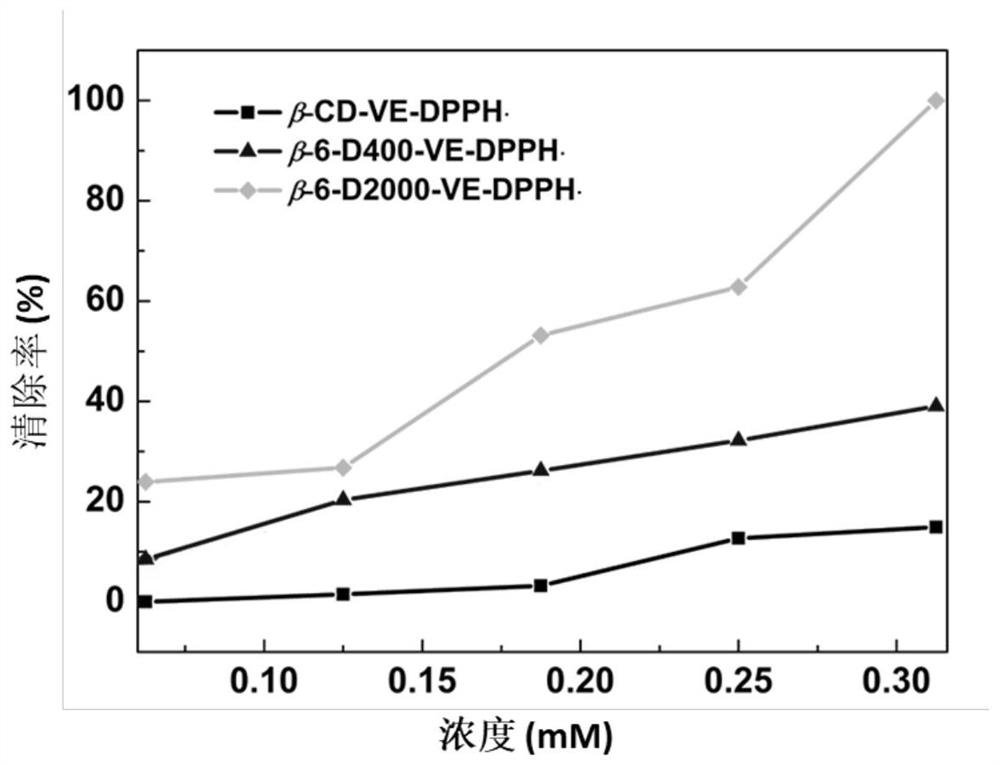
[0039] Determination of DPPH free radical scavenging rate of cyclodextrin and ether aminated cyclodextrin VE inclusion compound: Take 0.5, 1.0, 1.5, 2.0, 2.5mL of VE inclusion compound samples (1.00mM) respectively and place them in the colorimetric Then take 1.82mg DPPH and dilute it in a 100mL volumetric flask, configure 0.46mM ethanol solution, then pipette 5mL DPPH and dissolve it in the above colorimetric tube, then dilute to 10mL, dissolve evenly, and let it stand for 30min , Obtained the scavenging rate of DPPH free radicals of clathrate samples with different concentrations, and tested the ultraviolet-visible spectrum (U-3900, Hitachi, Japan), and calculated the scavenging rate.
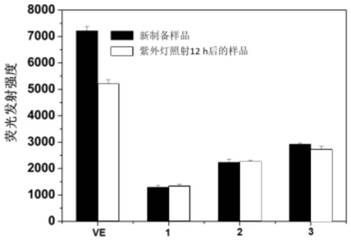
[0040] figure 1 The scavenging efficiency of host-guest inclusion complexes β-CD-VE, β-CD-6-D400-VE, β-CD-6-D2000-VE at different concentrations on DPPH·free radicals. Depend on figure 1 It can be seen that when the concentration of the clathrate is 0.35mM, it has the highest scavenging rat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com