A crystal form of pentostatin and its preparation method and application
A pentostatin and crystal form technology, applied in the field of chemical pharmacy, can solve the problems of unfavorable crystal form stability preparation storage stability, poor flowability of needle crystals, affecting preparation operability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
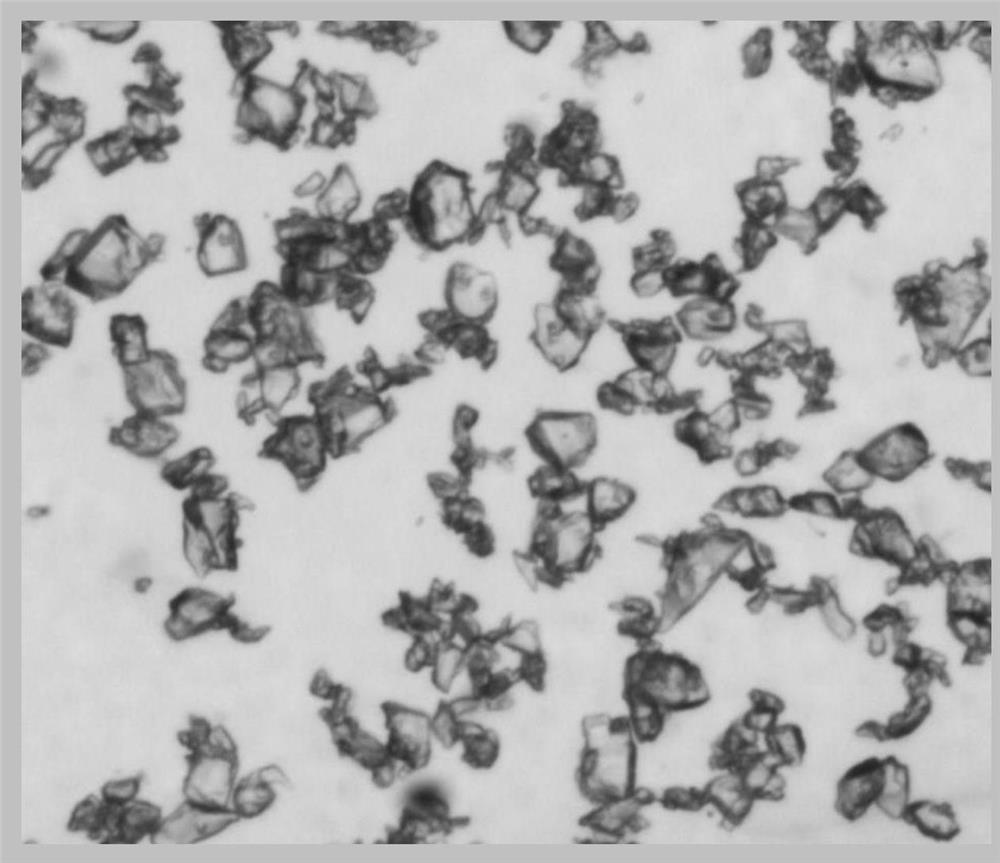
[0049] Dissolve 0.2g of crude pentostatin (HPLC purity>95%) in 20mL of methanol, heat up to 50°C, dissolve, filter, add 80ml of methyl acetate under stirring, crystallize at 20°C for 6h, control the stirring speed at 170rpm / min, filtered, and vacuum-dried at 35°C to obtain 0.08g blocky crystals, which were easy to filter and had a purity of 99.7% by HPLC.
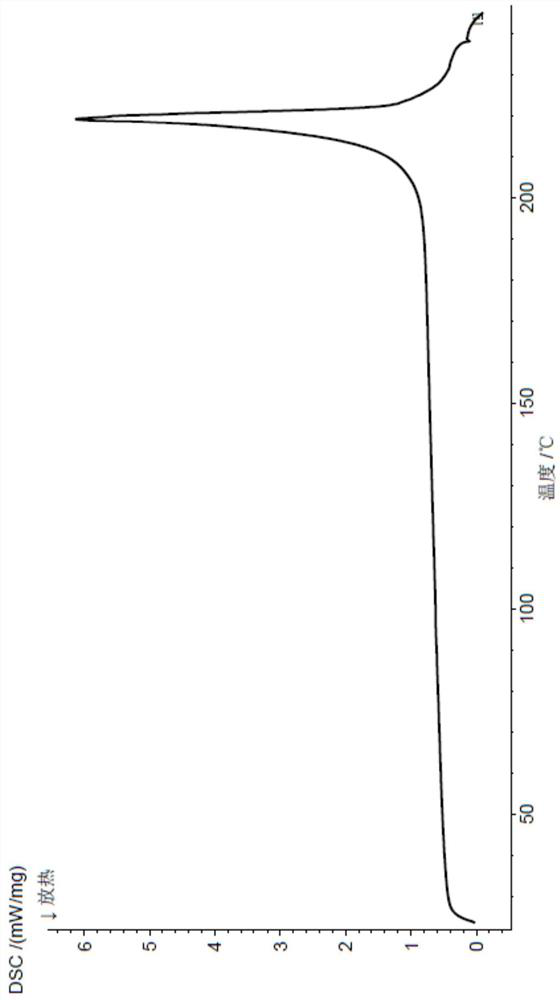
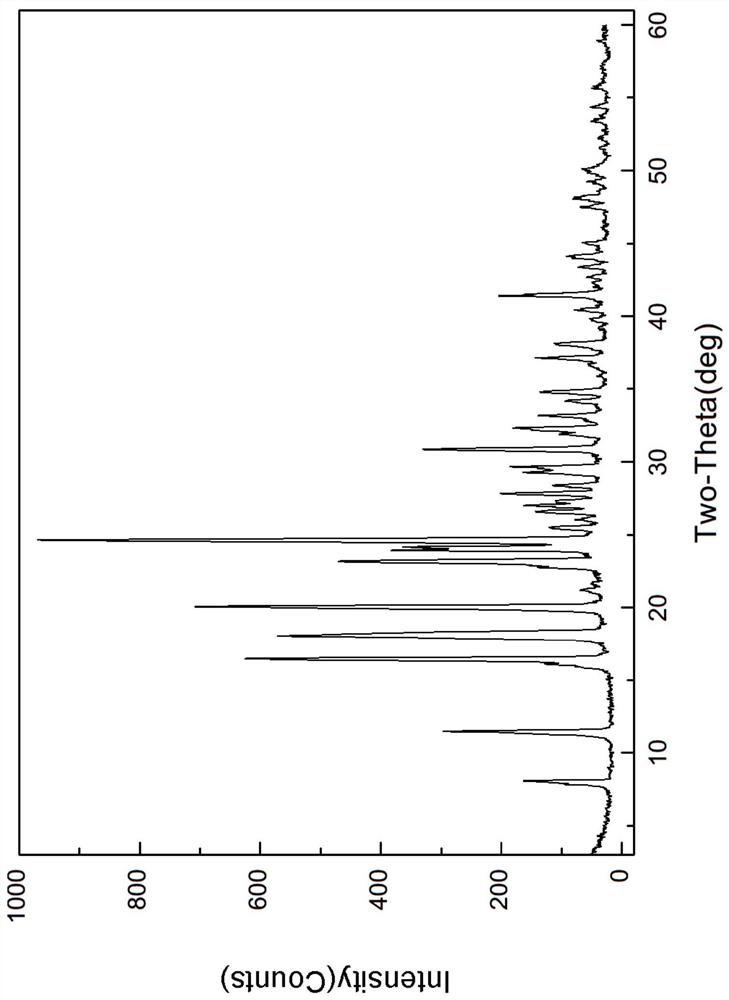
[0050] The X-ray powder diffraction and DSC spectrum of this crystal are detailed in Figure 1-2 , which is named pentostatin crystal form I in the present invention.
Embodiment 2
[0052] Dissolve 0.2g of crude pentostatin (HPLC purity>95%) in 10mL of methanol, heat up to 60°C, dissolve, filter, add 60ml of methyl acetate under stirring, crystallize at 25°C for 24h, control the stirring speed at 170rpm / min, filtered, and vacuum-dried at 35°C to obtain 0.11 g blocky crystals, which were easy to filter and had a purity of 99.5% by HPLC. According to X-ray powder diffraction pattern (XRD), it was confirmed to be pentostatin crystal form I.
Embodiment 3
[0054] Dissolve 0.2 g of crude pentostatin (HPLC purity >95%) in 12 mL of methanol, heat up to 55 ° C, dissolve, filter, add 120 ml of methyl acetate while stirring, crystallize at 30 ° C for 8 h, control the stirring speed at 170 rpm / min, filtered, and vacuum-dried at 35°C to obtain 0.14 g blocky crystals, which were easy to filter and had a purity of 99.1% by HPLC. According to X-ray powder diffraction pattern (XRD), it was confirmed to be pentostatin crystal form I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com