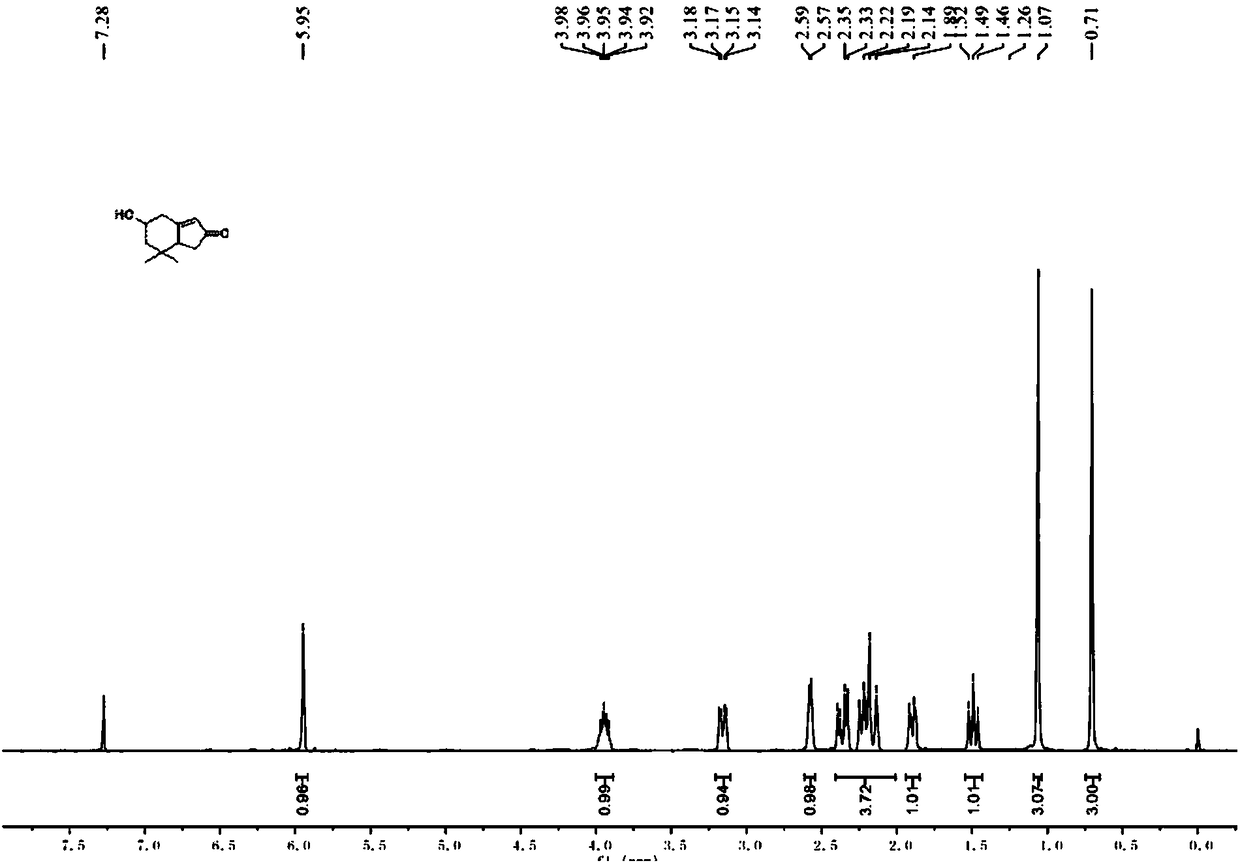
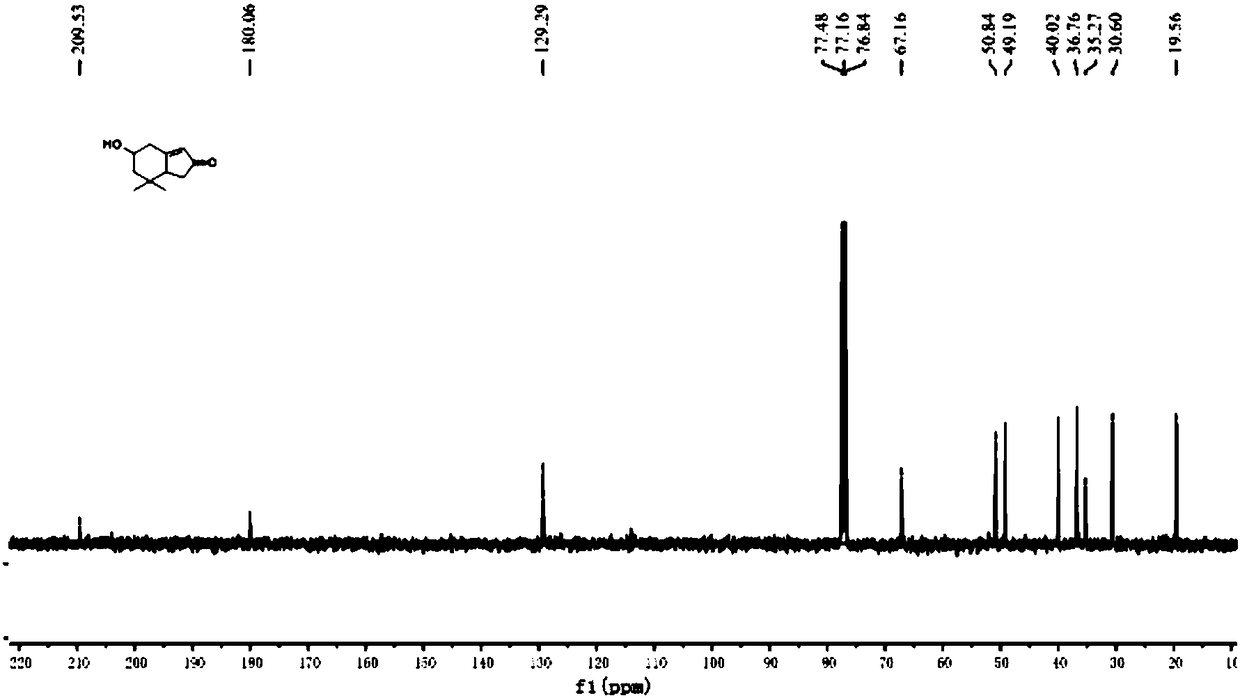
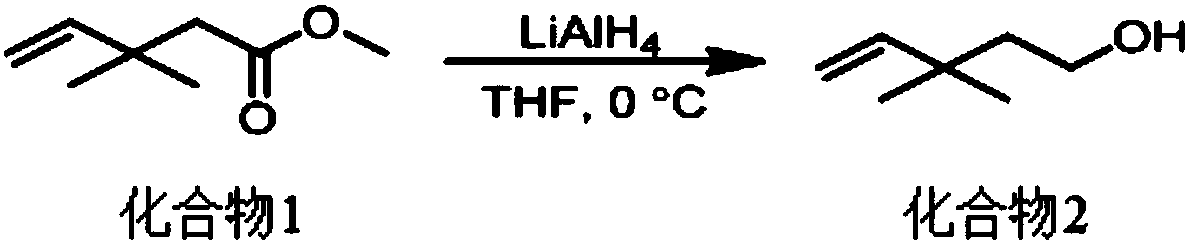Preparation method of 5-hydroxy-7,7-dimethyl-2H-indanone
A dimethyl and hydroxyl technology, applied in the field of natural product synthesis, achieves the effects of simple operation steps, simple preparation method and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]Step 1, under nitrogen protection, 2mol of lithium aluminum hydride (LiAlH 4 ) placed in an anhydrous and anaerobic treated eggplant bottle, then added redistilled tetrahydrofuran (THF) and stirred to make lithium aluminum hydride (LiAlH 4 ) tetrahydrofuran (THF) solution and cooled to 0 ° C; under nitrogen protection, 1mol of 3,3-dimethyl-4-pentenoic acid methyl ester was placed in an anhydrous and anaerobic treated eggplant bottle, and then Add redistilled THF and stir to make a THF solution containing 3,3-dimethyl-4-pentenoic acid methyl ester; Slowly drop the THF solution of methyl ester (the dropping speed is 5-20 drops / min) at 0°C containing lithium aluminum hydride (LiAlH 4 ) in tetrahydrofuran (THF) solution, after the reaction is complete, use saturated NH 4 Quenched with aqueous Cl solution, extracted with ethyl acetate, dried the organic phase with anhydrous sodium sulfate, concentrated and separated by column chromatography (the volume ratio of ethyl acetat...
Embodiment 2
[0049] Step 1, under nitrogen protection, 2mol of lithium aluminum hydride (LiAlH 4 ) placed in an anhydrous and anaerobic treated eggplant bottle, then added redistilled tetrahydrofuran (THF) and stirred to make lithium aluminum hydride (LiAlH 4 ) in tetrahydrofuran (THF) and cooled to -10°C; under nitrogen protection, 1 mol of 3,3-dimethyl-4-pentenoic acid methyl ester was placed in an anhydrous and anaerobic treated eggplant bottle, Then add redistilled THF and stir to make a THF solution containing 3,3-dimethyl-4-pentenoic acid methyl ester; Slowly drip the THF solution of methyl acrylate (the dropping speed is 5-20 drops / min) at 0°C containing lithium aluminum hydride (LiAlH 4 ) in tetrahydrofuran (THF) solution, after the reaction is complete, use saturated NH 4 Quenched with aqueous Cl solution, extracted with ethyl acetate, dried the organic phase with anhydrous sodium sulfate, concentrated and separated by column chromatography (the volume ratio of ethyl acetate to ...
Embodiment 3
[0054] Step 1, under nitrogen protection, 2mol of lithium aluminum hydride (LiAlH 4 ) placed in an anhydrous and anaerobic treated eggplant bottle, then added redistilled tetrahydrofuran (THF) and stirred to make lithium aluminum hydride (LiAlH 4 ) in tetrahydrofuran (THF) and cooled to -5°C; under nitrogen protection, 1 mol of 3,3-dimethyl-4-pentenoic acid methyl ester was placed in an anhydrous and anaerobic treated eggplant bottle, Then add redistilled THF and stir to make a THF solution containing 3,3-dimethyl-4-pentenoic acid methyl ester; Slowly drip the THF solution of methyl acrylate (the dropping speed is 5-20 drops / min) at 0°C containing lithium aluminum hydride (LiAlH 4 ) in tetrahydrofuran (THF) solution, after the reaction is complete, use saturated NH 4 Quenched with aqueous Cl solution, extracted with ethyl acetate, dried the organic phase with anhydrous sodium sulfate, concentrated and separated by column chromatography (the volume ratio of ethyl acetate to p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com