Method for testing ion diffusion coefficient in solid electrolyte
A solid electrolyte and ion diffusion technology, applied in the fields of chemistry, material science and engineering, and physics, can solve problems such as difficult to determine parameters, limited testing technology, and difficulty in applying electrolyte materials, and achieve the effect of easy realization and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
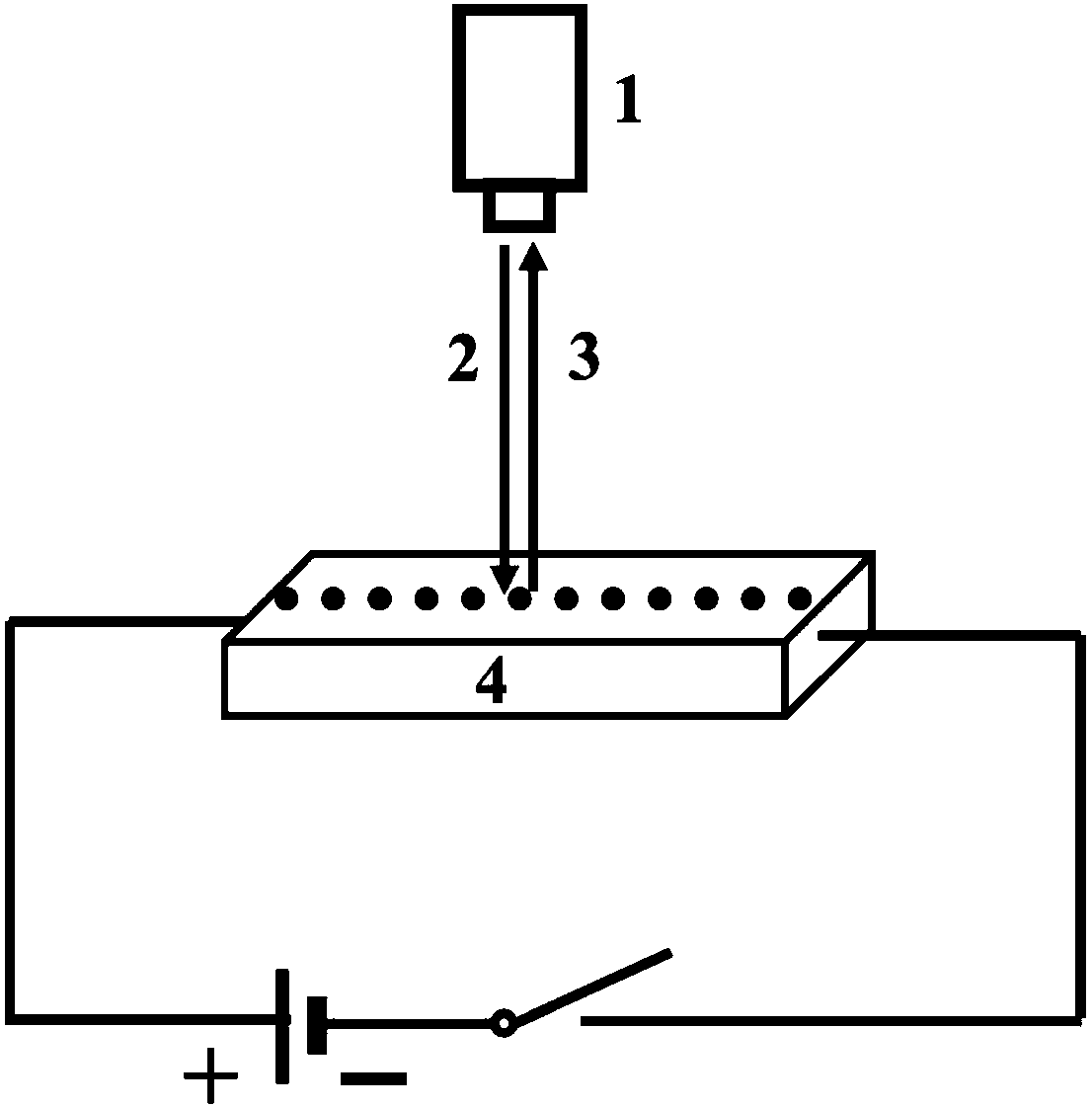
[0062] (1) The solid electrolyte Ce 0.65 La 0.35 o 1.825 A dense test sample is synthesized, cut into a rectangular cuboid, and after the surface is polished, silver paste is plated on two opposite end faces in the length direction as electrodes, and silver wires are glued on as leads. After the electrode part is coated with high-melting point glass powder, it is placed in a muffle furnace and heated to 800 ° C and then cooled with the furnace to seal the electrode part and insulate it from the air.
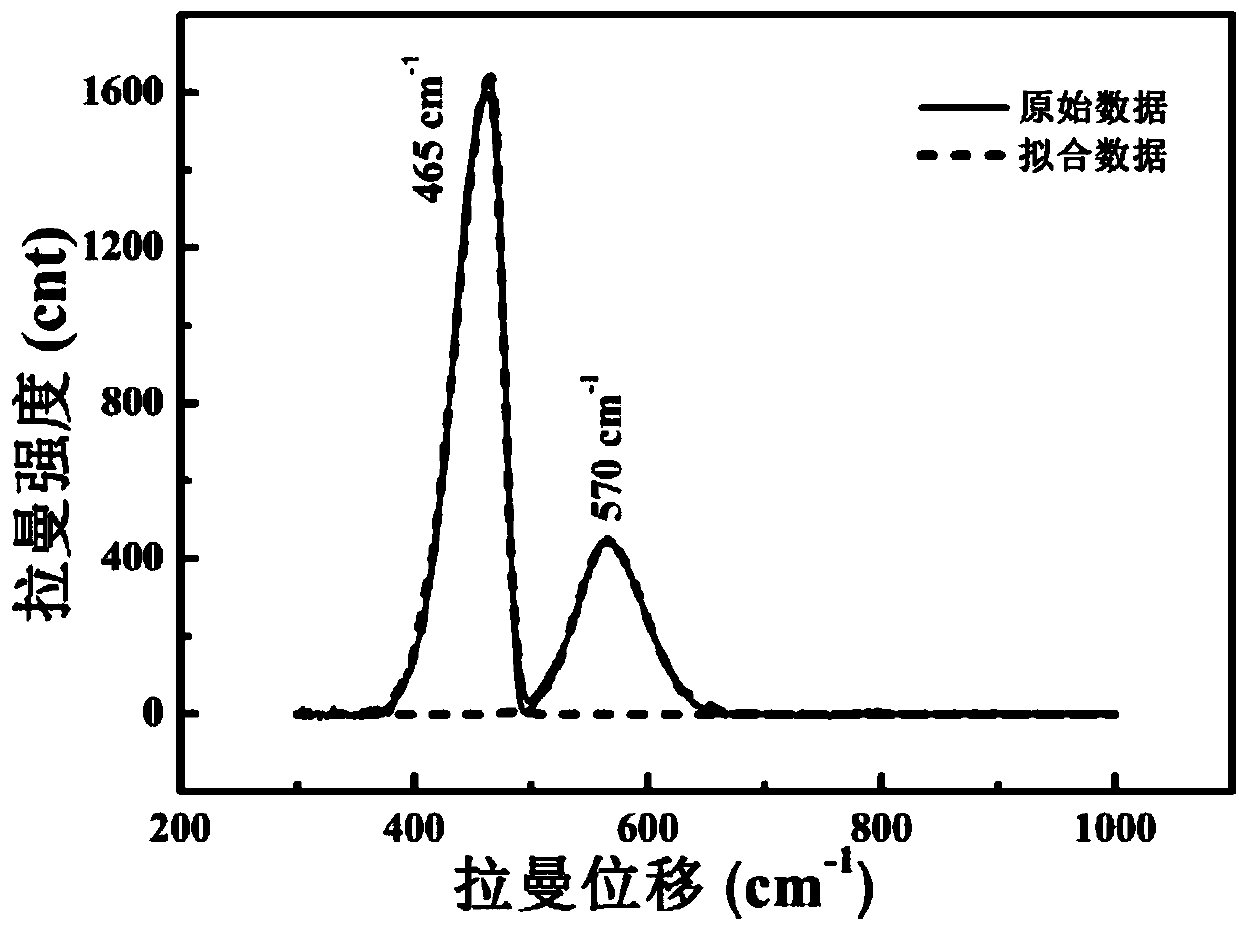
[0063] (2) Place the solid electrolyte in a heating table and heat it to a first temperature of 450° C., between two electrodes, start from the edge of one side of the electrode, and test the Raman spectrum of the material at intervals of 100 μm.
[0064] (3) Apply an electric field with an intensity of 54 V / m, and keep warm at the first temperature of 450° C. for 12 hours.
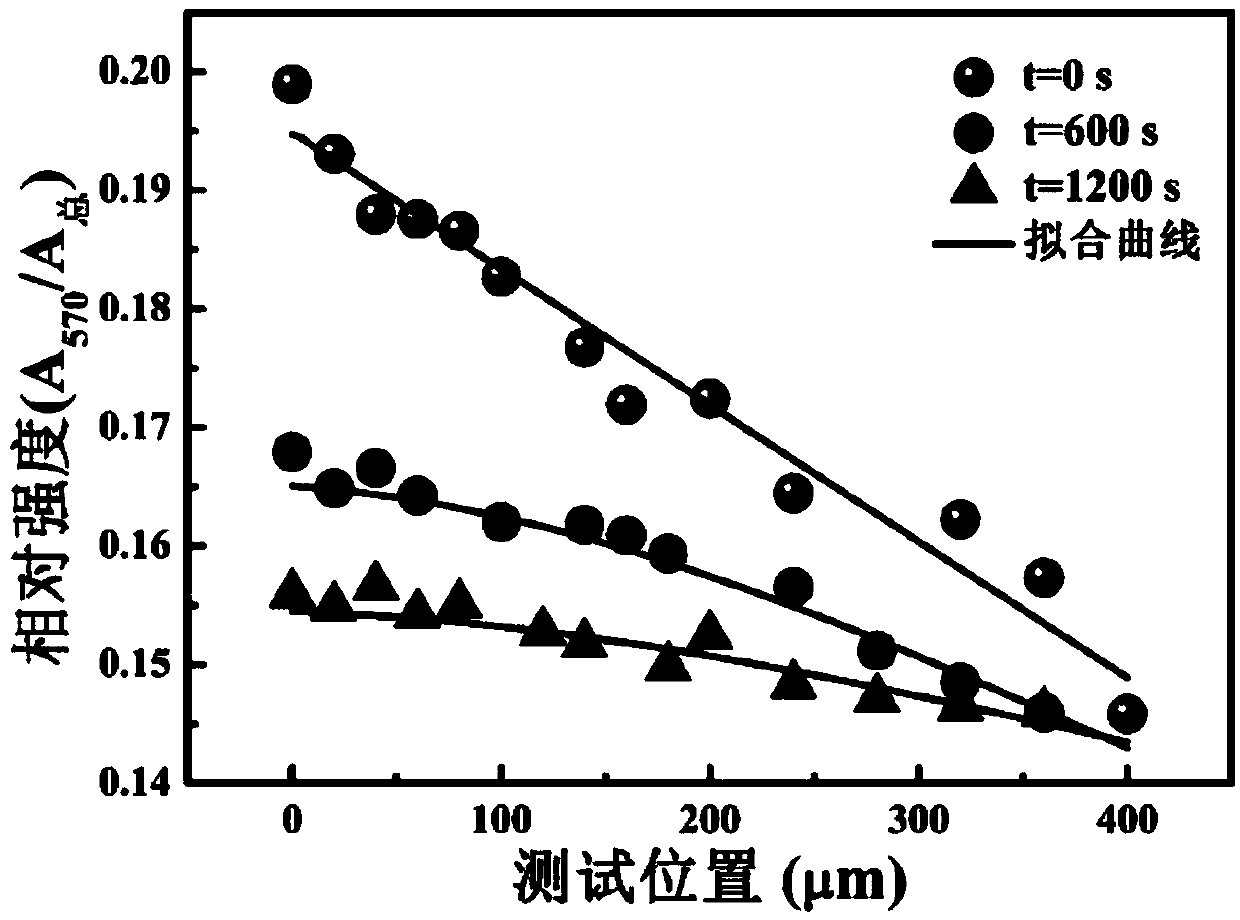
[0065] (4) remove the electric field, when t=0min, between the two electrodes, from the edge of the el...
Embodiment 2
[0069] (1) The solid electrolyte Ce 0.65 La 0.35 o 1.825 A dense test sample is synthesized, cut into a rectangular cuboid, and after the surface is polished, silver paste is plated on two opposite end faces in the length direction as electrodes, and silver wires are glued on as leads. After the electrode part is coated with high-melting point glass powder, it is placed in a muffle furnace and heated to 800 ° C and then cooled with the furnace to seal the electrode part and insulate it from the air.
[0070] (2) Place the solid electrolyte in a heating table and heat it to the first temperature of 450°C. Between the two electrodes, start from the edge of the electrode on one side, and test the Raman spectrum of the material at intervals of 100 μm.
[0071] (3) Apply an electric field with an intensity of 54 V / m, and keep warm at the first temperature of 450° C. for 12 hours.
[0072] (4) remove the electric field, when t=0min, between the two electrodes, from the edge of th...
Embodiment 3
[0076] (1) The solid electrolyte Ce 0.65 La 0.35 o 1.825 A dense test sample is synthesized, cut into a rectangular cuboid, and after the surface is polished, silver paste is plated on two opposite end faces in the length direction as electrodes, and silver wires are glued on as leads. After the electrode part is coated with high-melting point glass powder, it is placed in a muffle furnace and heated to 800 ° C, and then cooled with the furnace to seal the electrode part and insulate it from the air;
[0077] (2) Put the solid electrolysis in the heating table and heat it to the first temperature of 500°C. Between the two electrodes, start from the edge of the electrode on one side, and test the Raman spectrum of the material at intervals of 100 μm;
[0078] (3) Apply an electric field with an intensity of 54V / m, and keep it warm at a first temperature of 500°C for 10 hours;
[0079] (4) remove the electric field, when t=0min, between the two electrodes, from the edge of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com