A kind of detection method utilizing fluorescence sensing p-trinitrotoluene
A trinitrotoluene and detection method technology, which is applied in the field of explosive detection, can solve the problems of poor solubility, complex synthesis method, sensitivity and repeatability to be developed, and achieve high solubility and high fluorescence quenching efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
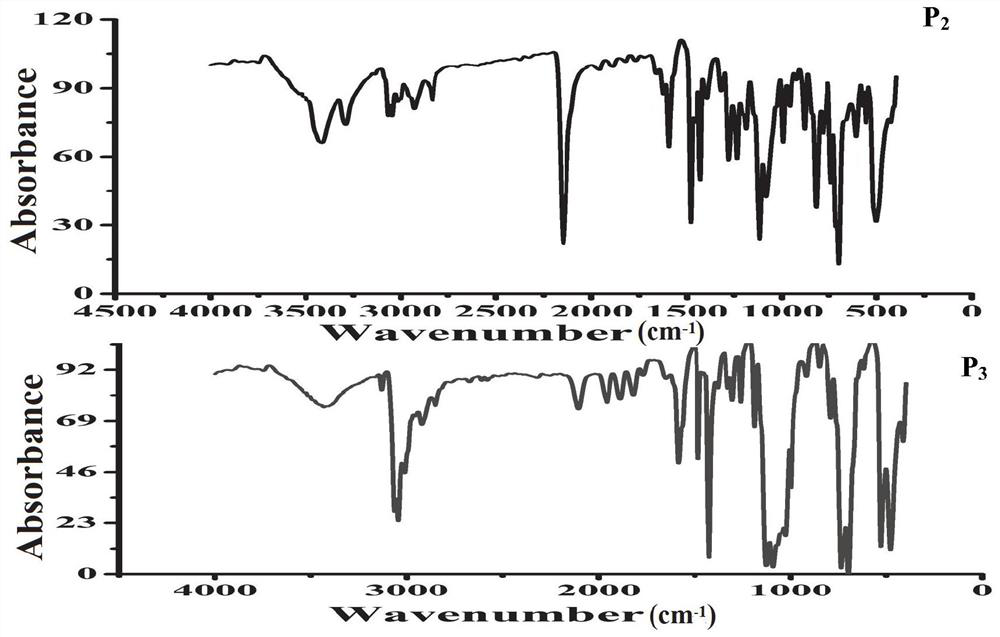
[0024] A typical implementation of the present application provides an application of a silicon-containing alkynyl carbazole hyperbranched polymer in the fluorescence detection of trinitrotoluene. The chemical structural formula of the silicon-containing alkynyl carbazole hyperbranched polymer is :
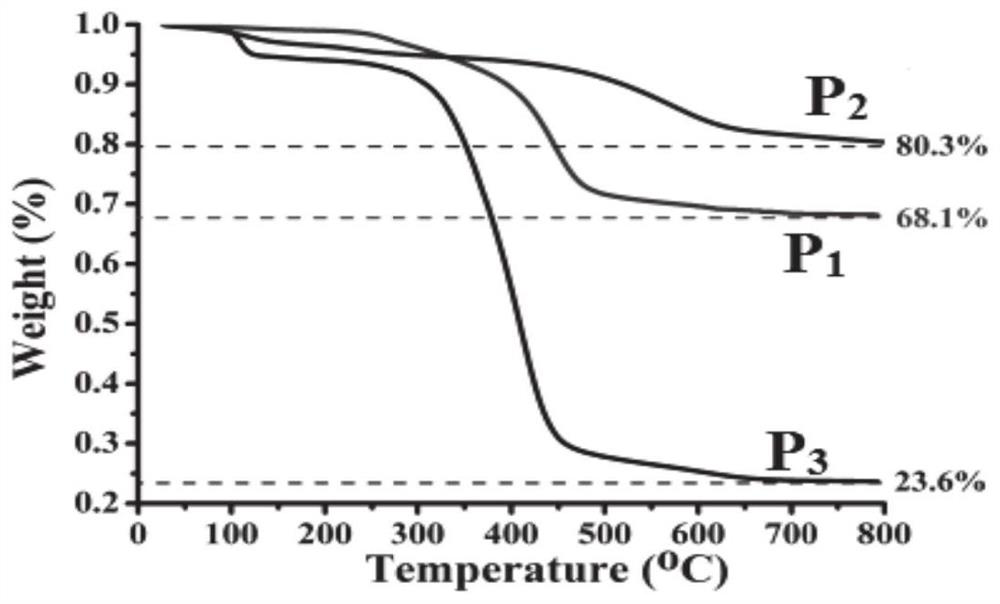
[0025] The weight average molecular weight of the hyperbranched polymer is 4800-5100 g / mol.
[0026] Preferably, the polydispersity index (PDI) of the hyperbranched polymer is 1.5-1.6.
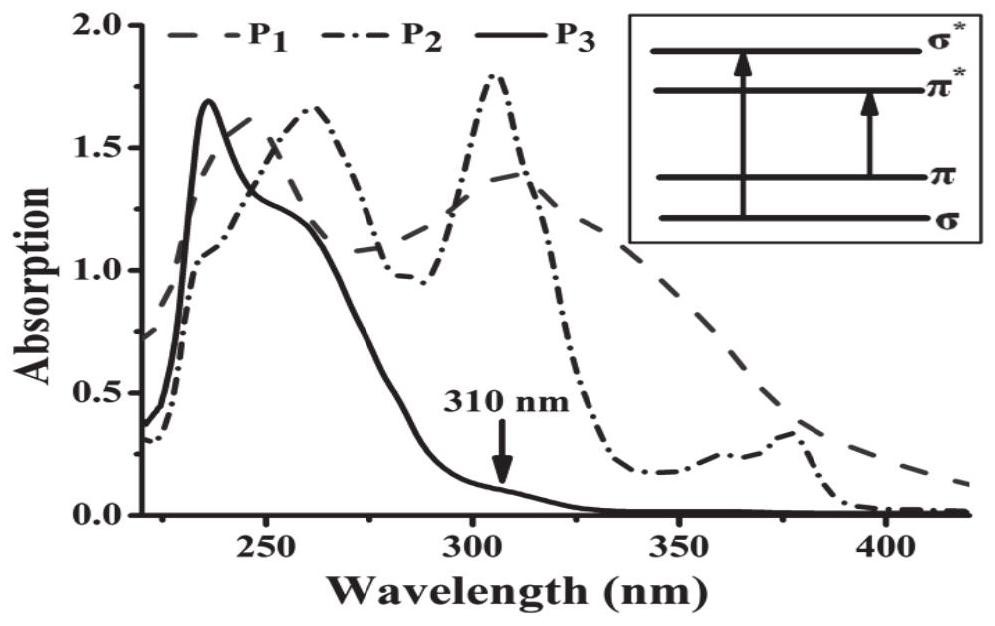
[0027] Another embodiment of the present application provides a method for detecting p-trinitrotoluene using fluorescence sensing, adding 2,4,6-trinitrotoluene to a silicon-containing alkynyl carbazole hyperbranched polymer The solution to be tested is obtained in the solution, and the fluorescence intensity of the solution to be tested is detected. The chemical structural formula of the alkynyl carbazole hyperbranched polymer containing silicon is:
[0028] The weight average molecular weig...
Embodiment 1
[0042] Add carbazole (3.09g, 18.6mmol) into the round bottom flask, pour 100mL of glacial acetic acid, stir at reflux temperature until the carbazole is completely dissolved, then add KI (4.98g, 10mmol), KIO 3 (3.9g, 18.0mmol), potassium iodate was added three times, stirred and refluxed for 6h. After the time is up, precipitates gather on the flat bottom, and the reaction solution is cooled to room temperature and then filtered. The resulting solid is washed with dichloromethane and then with methanol. After drying, it becomes a light pink solid powder, that is, 1,3,6- Triiodocarbazole. Yield 70%. 1 H-NMR:(DMSO,400MHz),δ(ppm):7.401-7.403(d,1H),7.689-7.713(m,1H),8.025(s,1H),8.566-8.509(d,2H),11.439 (s,1H).
[0043] Take 1,3,6-triiodocarbazole (2g, 4.55mmol) into a three-necked flask, add 20mL tetrahydrofuran, 10mL triethylamine, stir to dissolve, add trimethylsilylacetylene (3.57g, 36.36mmol), C 18 h 15 P (61mg, 0.56mmol), CuI (50mg, 0.27mmol), after bubbling argon for 2...
Embodiment 2
[0046] In a 100mL flask, add triacetylcarbazole (0.5g, 2.09mmol), 3,6-dibromo-9-hexylcarbazole (3g, 10mmol), triethanolamine (10mL), toluene (30mL), mix well, and argon Gas protection for 30min, then add [(C 6 h 5 ) 3 P] 2 PdCl 2 (58mg, 0.05mmol), CuI (10mg, 0.05mmol), under the protection of argon, stirred and refluxed for 48 hours. After the reaction stopped, it was cooled to room temperature, the solution was obtained by suction filtration, and the solvent was spin-dried to obtain a crude product. The crude product was extracted with ethyl acetate Soxhlet for 24 hours to remove unnecessary small molecules to obtain the final brown solid product, denoted as P 1 , Yield: 80%. 1 H NMR: (CDCl 3 ,400MHz),δ(ppm):0.27-0.92(s,12H),0.98-1.46(d,16H),1.50-1.71(s,8H),1.73-2.02(s,2H),7.31-8.63(m ,14H).
[0047] The chemical formula of its preparation process is as follows:
[0048]
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com