Homogeneous immunoassay kit for detecting β-human chorionic gonadotropin and its preparation method and application
A chorionic gonadotropin and immune detection technology, applied in the field of immune analysis, can solve the problems affecting the accuracy and sensitivity of the analysis method, the precision of the analysis method, and the washing error of the immune analysis, etc., and achieves a wide range of signal values and sensitivity. High and satisfactory analytical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: Preparation of a homogeneous immunoassay reagent kit for detecting β-human chorionic gonadotropin
[0078] (1) Preparation of receptors bound to antibodies:
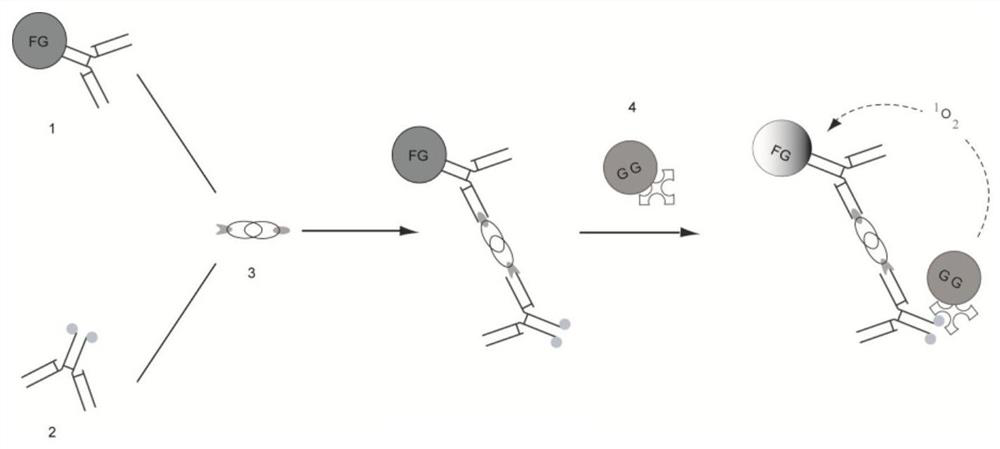
[0079] The acceptor used in this example was microparticles (luminescent microspheres) containing aldehyde groups (-CHO) coated on latex particles to form chelates filled with derivatives of dimethylthiophene and lanthanide Eu. The receptor is attached to the antibody molecule through an aldehyde group.
[0080] Biological material: β-hCG monoclonal antibody
[0081] Preparation process: take 2 mg of luminescent microsphere solution and dilute it to 5 mg / ml with 0.05M carbonate buffer (CB) pH 9.6; transfer 0.02 mg of β-hCG monoclonal antibody to the diluted luminescent microsphere solution Mix well, overnight at 4°C; then add 20uL of BSA solution diluted to 10mg / ml with 0.05M CB buffer at pH 9.6, rotate at room temperature for 2h; wash the microspheres thoroughly, and finally use 0.1M Tris-HCl at pH 8...
Embodiment 2
[0094] Example 2: Detection of β-human chorionic gonadotropin in a sample using the reagent kit prepared by the method of the present invention.
[0095] The reagent set used is the homogeneous immunodetection reagent set for β-human chorionic gonadotropin prepared in Example 1. The detection process is automatically completed by the automatic photo-excited chemiluminescence analysis system and the detection results are output. The specific steps are as follows:
[0096] 1) Add 10 μl of the sample to be tested or calibrator and quality control product to the reaction wells;
[0097] 2) Add 25 μl R1 and 25 μl R1 to the reaction well in turn;
[0098] 3) Incubate at 37°C for 15 minutes;
[0099] 4) Add 175 μl R3;
[0100] 5) Incubate at 37°C for 15 minutes;
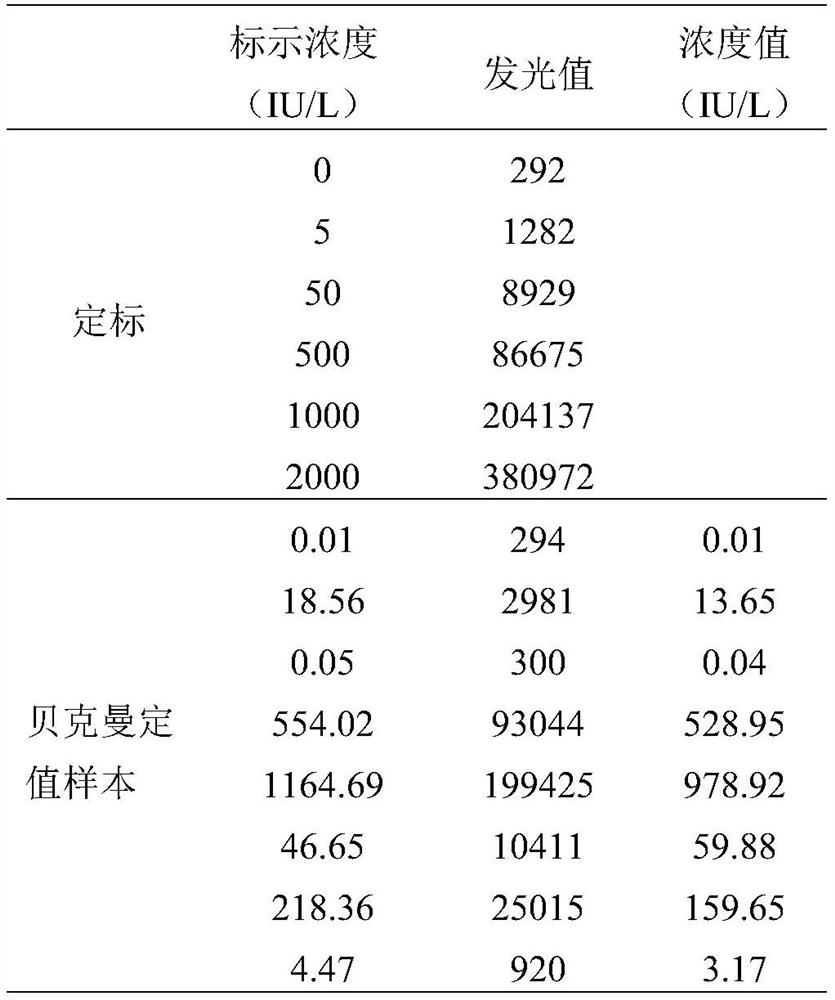
[0101] 6) Irradiate the microwell with laser and calculate the signal value of each well; draw a standard curve according to the signal value of the calibrator, and calculate the concentration of β-human chorionic gonad...
Embodiment 3
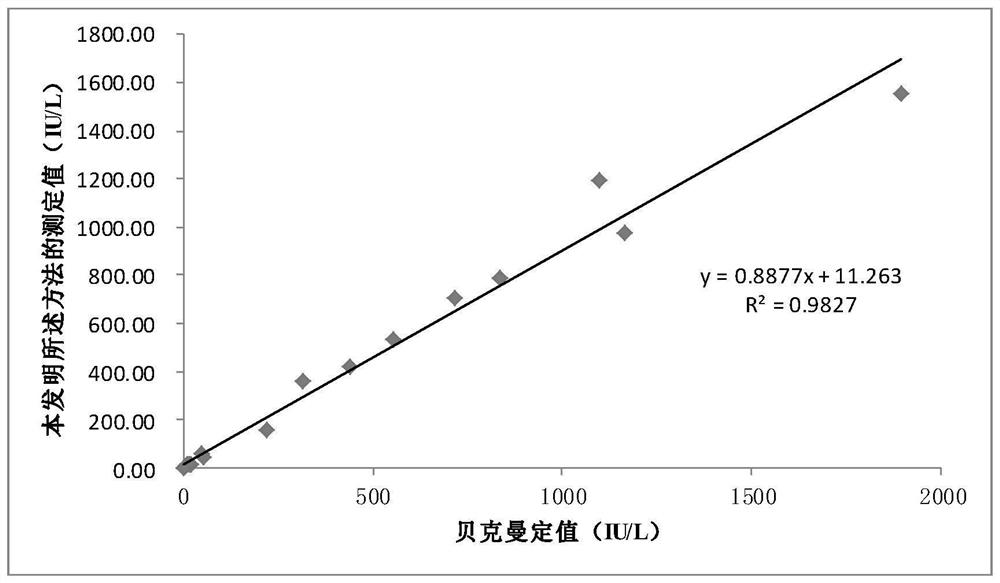
[0110] Example 3: Detection precision of the reagent kit prepared by the method of the present invention
[0111] Precision is an important indicator to measure the intra- and inter-assay variability of in vitro diagnostic reagents, and is an important basis for evaluating the effectiveness of products to be marketed, usually including intra-assay precision and inter-assay precision.
[0112] Intra-assay precision evaluation method: use samples with low (L), medium (M), and high (H) values to independently analyze 3 batches of products, and repeat the determination 10 times for each batch (using the method in Example 2). method described), calculate the average of 10 measurements and standard deviation (SD), according to the formula The coefficient of variation (CV) was calculated and the results are shown in Table 4.
[0113] Inter-batch precision evaluation method: use samples with low (L), medium (M), and high (H) values to independently analyze 3 batches of product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com