Application of tristilbeside in the preparation of medicines for treating and/or preventing inflammatory bowel disease
A technology of trizaperidin and inflammatory bowel disease, which is applied in the application field of medicine, can solve the problems such as the use of inflammatory bowel disease medicine that has not been reported, so as to reduce colonic mucosal congestion, improve bloody stool, increase weight and activity sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
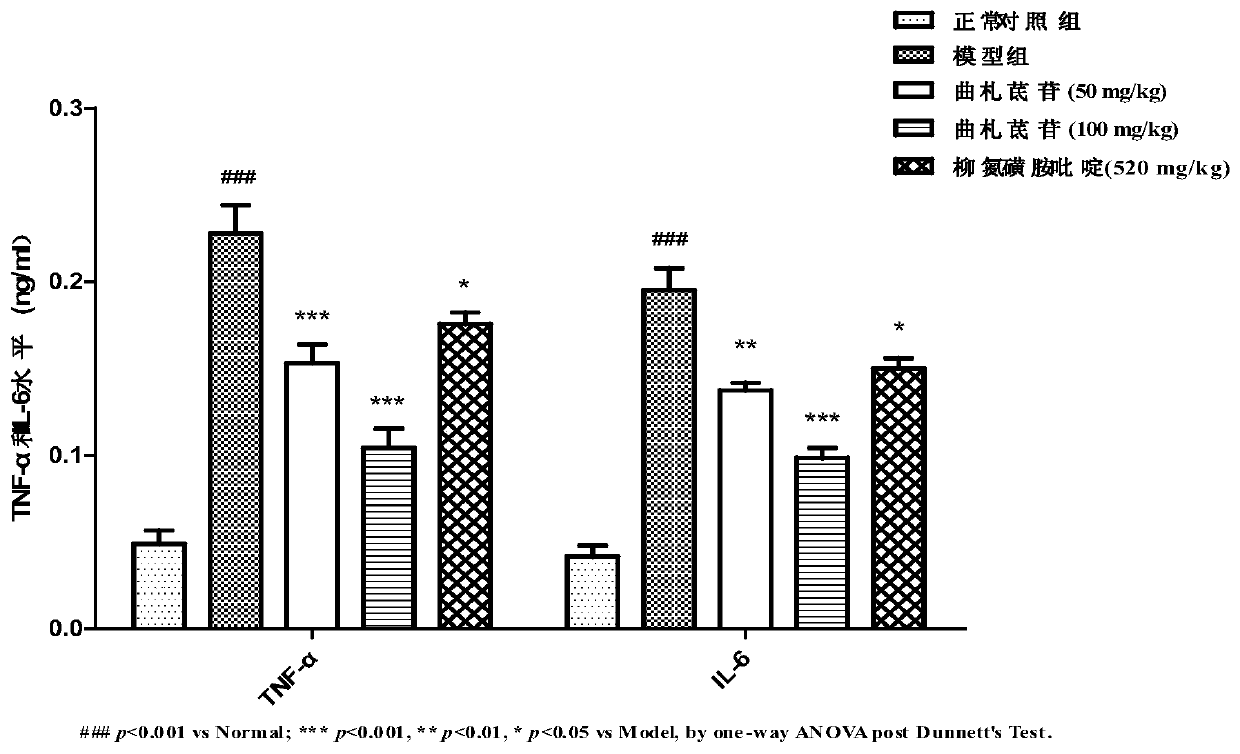
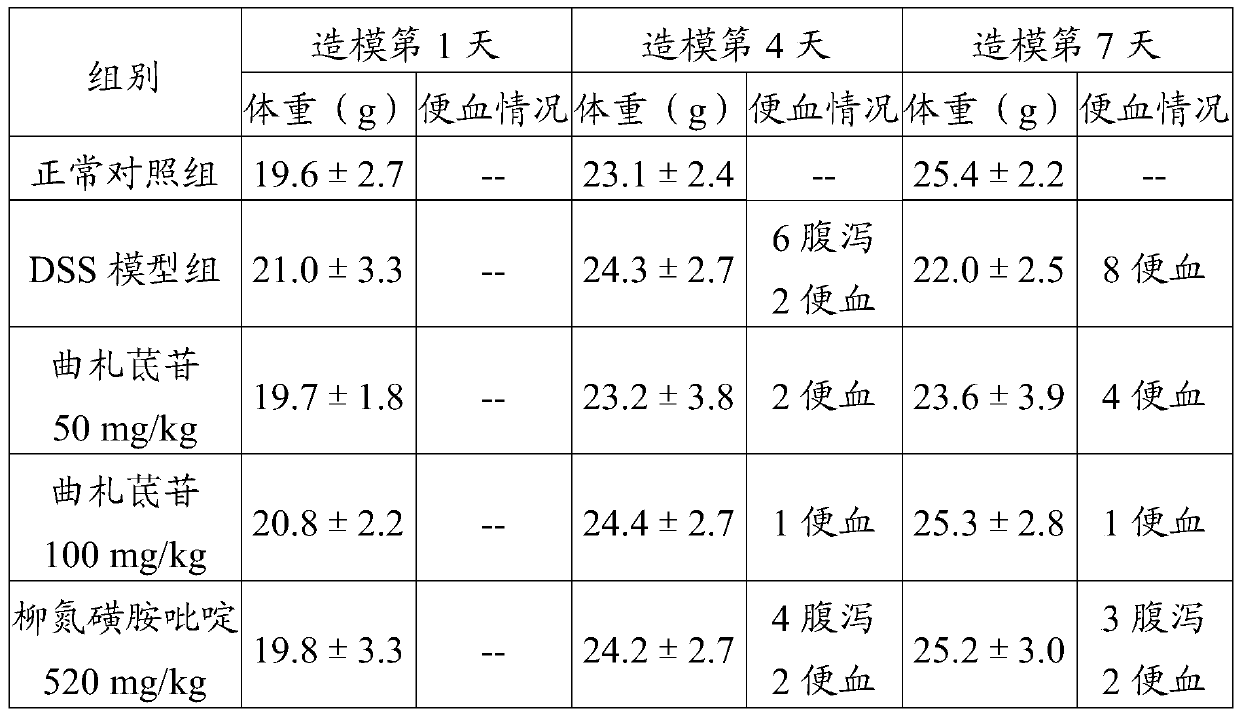
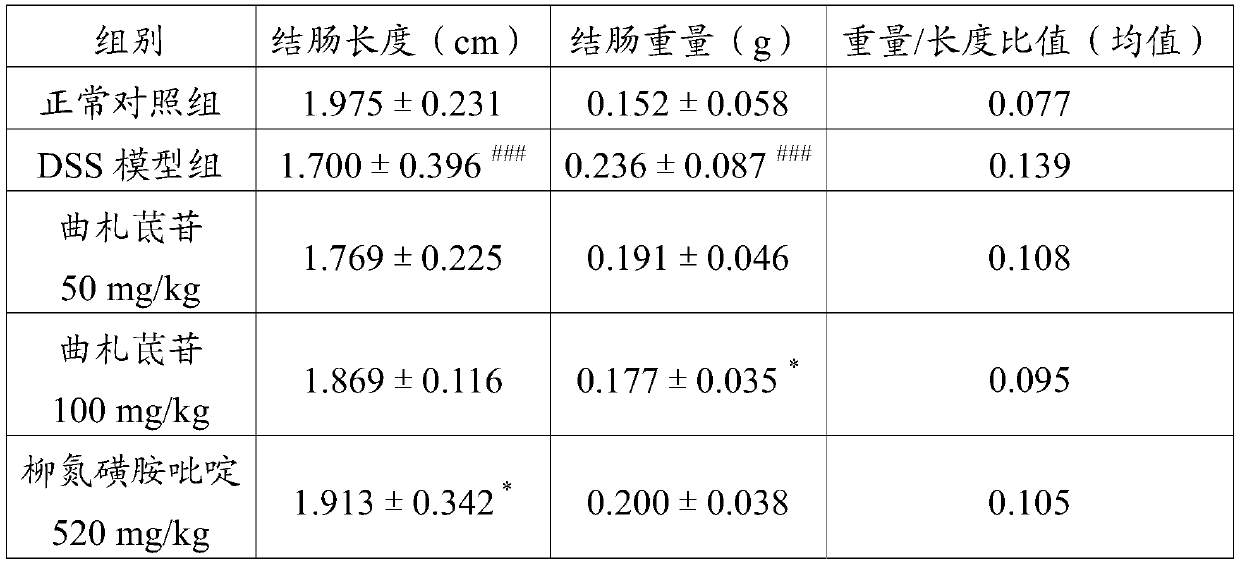
[0022] The efficacy test proved the preventive effect of trizaperidin on the mouse ulcerative colitis model:
[0023] 1 Reagents and Animals
[0024] 1.1 Test product: Tristilbene raw material, Kunming Pharmaceutical Group Co., Ltd., traits: white powder; batch number: 20150407; storage conditions: room temperature, dark;
[0025] 1.2 Reference substance: sulfasalazine (SASP, the earliest salicylic acid preparation used in the treatment of IBD), Meilun Biology, traits: yellow powder; batch number: A0322A; storage conditions: room temperature, dark;
[0026] 1.3 Other reagents: dextran sodium sulfate (DSS) was purchased from MP Biomedicals.LLC (France); sodium carboxymethylcellulose was purchased from Tianjin Guangfu Institute of Fine Chemicals; mouse TNF-αELISA detection kit (Lianke Biological , Lot: 228271215); mouse IL-6 ELISA detection kit (Lianke Bio, Lot: 220680144);
[0027] 1.4 Experimental animals: SPF grade BALB / c mice, 40 males, body weight: 18±2g, provided by Shan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com