Application of myricanol and/or myricetin to preparation of medicine for preventing and/or treating inflammatory bowel diseases
A technology for inflammatory bowel disease and myricetone, which is applied in the field of medicine, can solve the problems of unmet medical needs, low patient acceptance, and poor curative effect, and achieves the advantages of large-scale production, reduction of production costs, and reduction of ulcer area. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
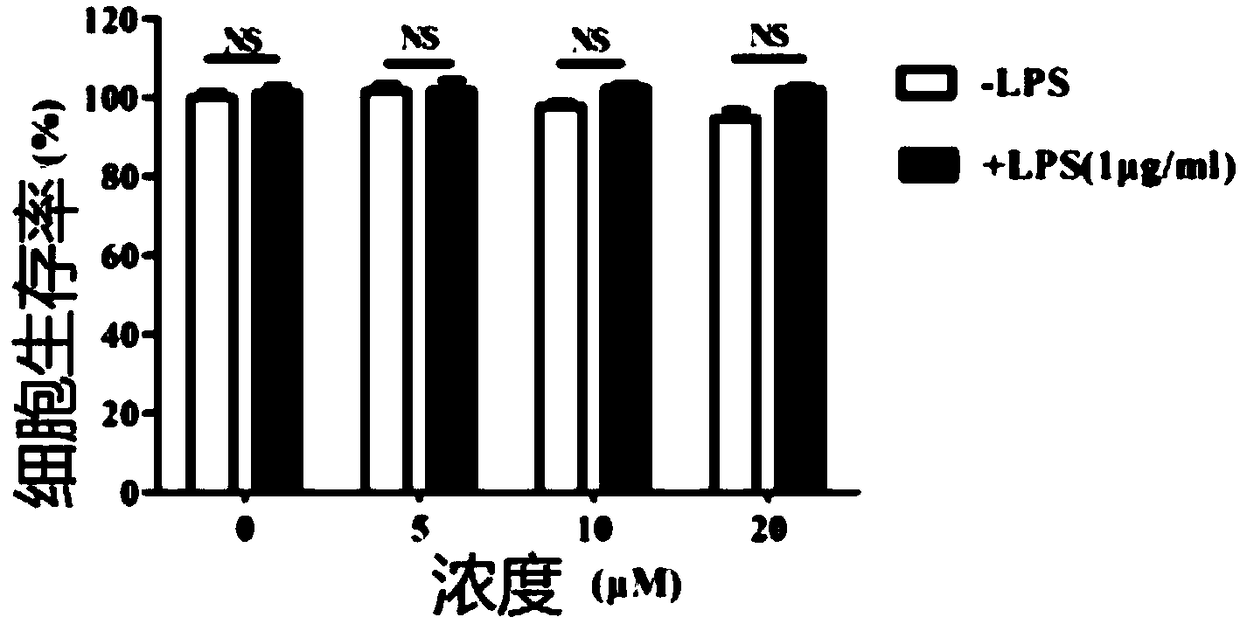
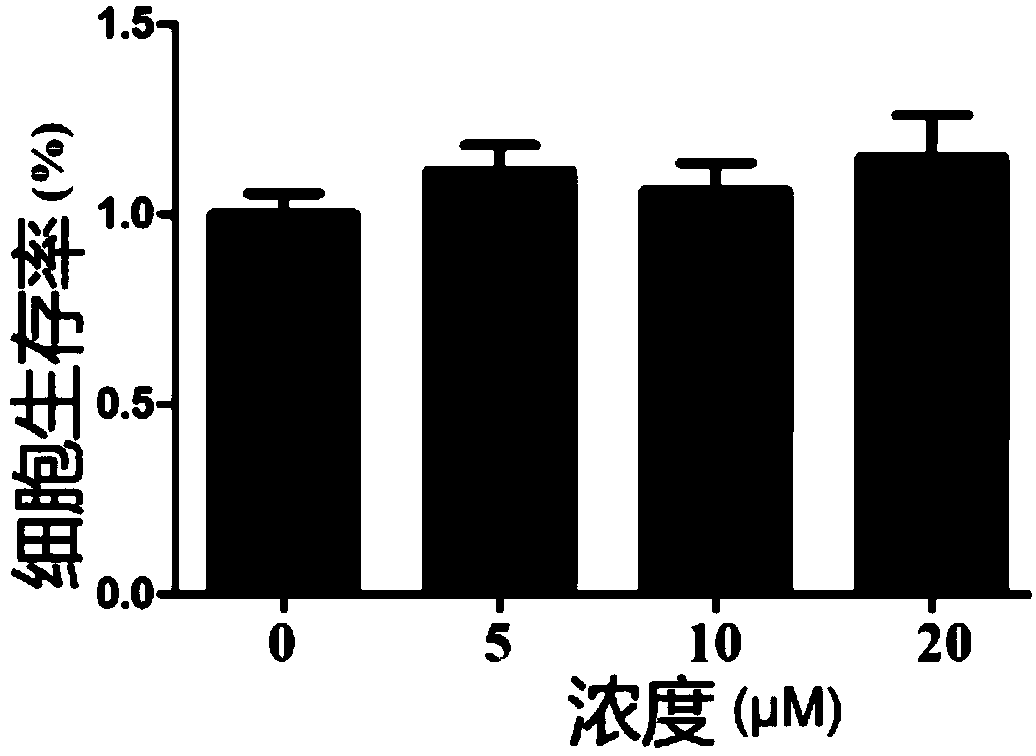
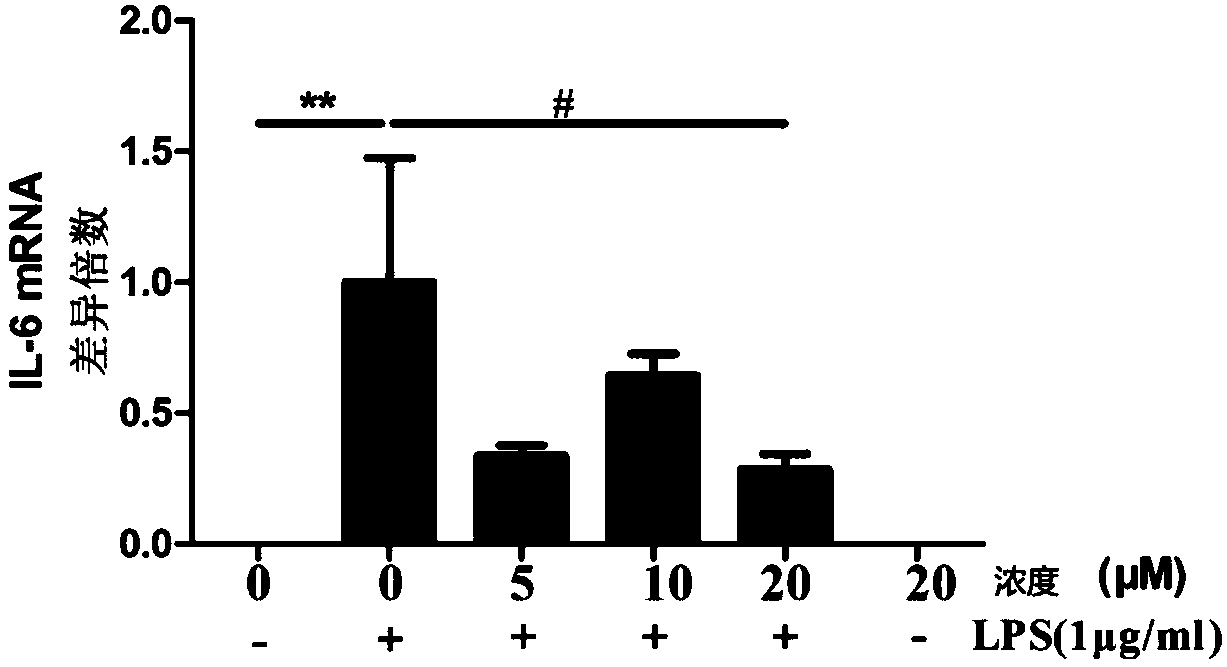
[0051] In this example, myricetin and myricetin, which are anti-inflammatory bowel disease drugs, were tested for anti-inflammatory activity in vitro
[0052] The effect of myricetin and myricetin on the expression of IL-6, TNF-α, IL-1β and other inflammatory factors secreted by the mouse macrophage cell line RAW264.7 induced by bacterial lipopolysaccharide (hereinafter referred to as LPS) stimulation: take pre-prepared The cell suspension was inoculated into a 96-well plate at 20,000 cells / well, 100 μL / well, and placed at 37°C, 5% CO 2 Cultivate overnight in the incubator, discard the original culture medium, add 100 μL of prepared medium with different concentrations of the test drug solution to each well, and add LPS after 1 hour of culture, and the final concentration of LPS in the culture system is 100 μg / mL. Three parallel wells were set for each concentration, and a cell control group (no drug, no solvent) and a blank medium control group (no cells, zero wells) were set...
Embodiment 2
[0057] In this example, an acute toxicity test was carried out on myricetin and myricetin, which are anti-inflammatory bowel disease drugs, to test the safety of myricetin and myricetin
[0058] Thirty SD rats were used, half male and half male. The average body weight of the group was 160-200 g for females and 180-220 g for males, and the individual body weights should be within the range of ±20% of the average body weight. Animals need to adapt to the environment for at least 5 days before the test, and healthy (female must not be pregnant) rats are selected as test animals. The main inspection contents during the adaptation period: whether it is consistent with the quality index required at the time of order; general status inspection; whether the weight reaches the weight range required by the test. Unqualified abnormal animals were not included in this experiment. Rats were intragastrically given a single dose, given low, medium and high doses, respectively 100mg / kg, 300...
Embodiment 3
[0064] In this example, the pharmacodynamics of myricetin as an anti-inflammatory bowel disease drug was tested in vivo on the IBD model in mice induced by dextran sodium sulfate (DSS)
[0065] SPF level C57BL / 6 mice, female, 6-8 weeks old, after 3-7 days of adaptive feeding, were fed with 4% dextran sodium sulfate (DSS) aqueous solution, and fed with free drinking water (day0 ), replace fresh DSS aqueous solution every 2 days, and after drinking DSS aqueous solution freely for 7 days (day7), replace fresh purified water for feeding. After 4 days of modeling, administration began on the 5th day of modeling, and the animals were given 10 mg / kg, 5 mg / kg, and 2.5 mg / kg of drugs for treatment through tail vein administration. The administration was continued for 4 days, followed by observation for 1 day, and the experiment was ended 24 hours after the last administration.
[0066] Detection index: observe and record the weight of animals in each group every day, and collect the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com