Bexarotene soft capsule and preparation method thereof
A technology of soft capsules and soft capsule shells, applied in the field of medicine, can solve the problems of poor dosage uniformity, difficulty in removing oxygen, poor fluidity, etc., and achieve the effect of simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
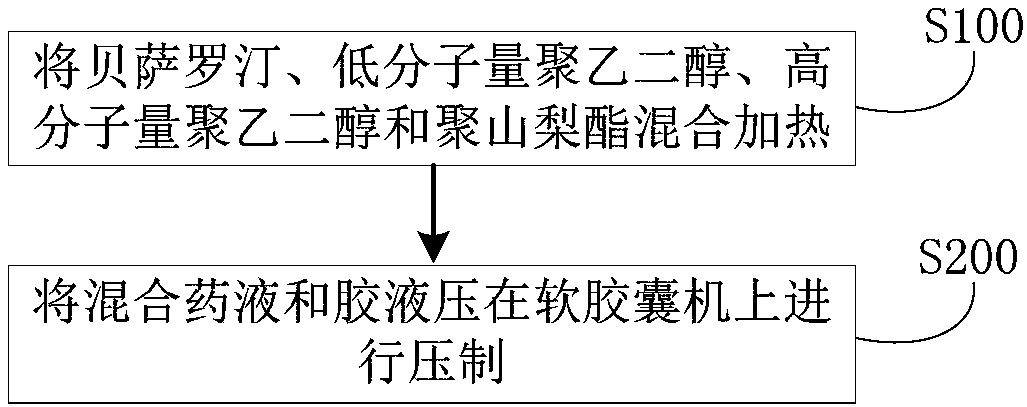
[0058] (1) Content composition: bexarotene 75g (D90 less than 20 microns), polyethylene glycol 400 450g, polyethylene glycol 2000 75g, polysorbate 20 60g.
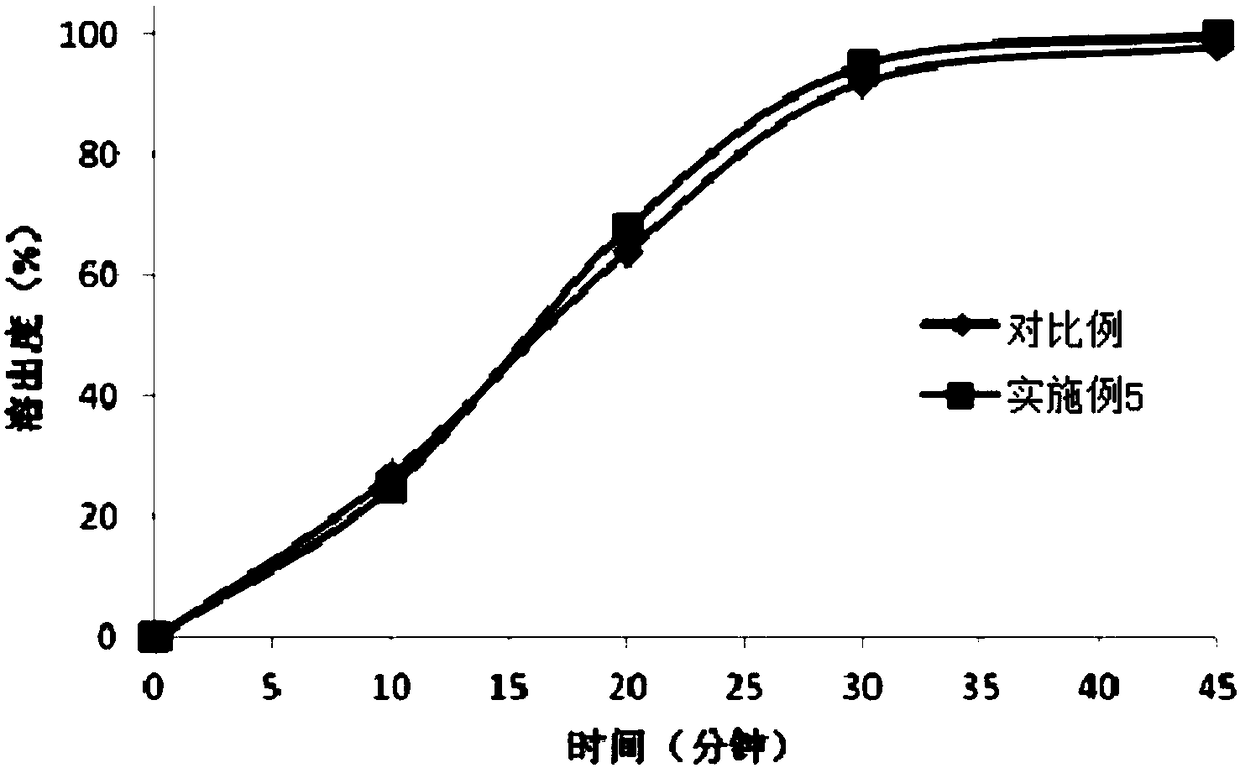
[0059] (2) Preparation method: first heat polyethylene glycol 400 to 60-80 degrees Celsius, then add polyethylene glycol 2000 and polysorbate 20 to it, stir, cool to 40-45 degrees Celsius after dissolving, and then pour in Add bexarotene and stir evenly to obtain a mixed liquid medicine. Next, keep the mixed medicinal solution at 30-35 degrees Celsius, and then press it with the glue solution (composed of gelatin, glycerin, sorbitol, water and titanium dioxide) on a soft capsule machine, and obtain bexarotene soft capsule after drying, polishing and packaging. capsule. The production process data are shown in Table 1.
Embodiment 2
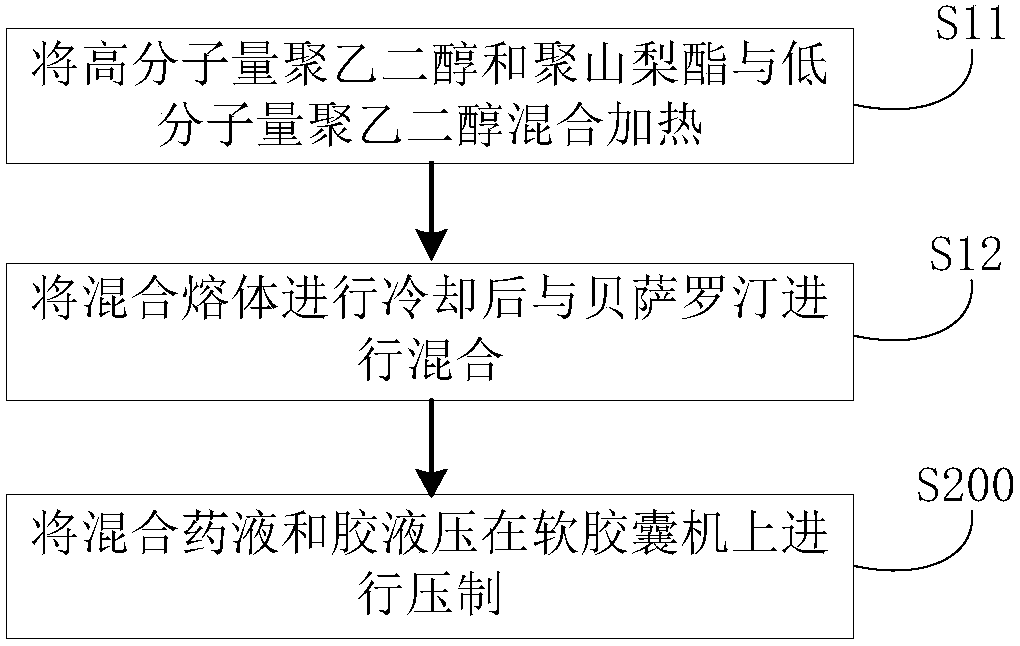
[0061] (1) Content composition: bexarotene 75g (D90 less than 20 microns), polyethylene glycol 600 600g, polyethylene glycol 1000 75g, polysorbate 40 45g.
[0062] (2) Preparation method: first heat polyethylene glycol 600 to 60-80 degrees Celsius, then add polyethylene glycol 1000 and polysorbate 40 to it, stir, cool to 40-45 degrees Celsius after dissolving, and then pour in Add the bexarotene and mix well. Keep the mixed medicinal liquid at 30-35 degrees Celsius, and then press it with the glue (composed of gelatin, glycerin, sorbitol, water and titanium dioxide) on a soft capsule machine, and obtain bexarotene soft capsules after drying, polishing and packaging . The production process data are shown in Table 1.
Embodiment 3
[0064] (1) Content composition: bexarotene 75g (D90 less than 20 microns), polyethylene glycol 400 300g, polyethylene glycol 1000 37.5g, polysorbate 40 75g.
[0065] (2) Preparation method: first heat polyethylene glycol 400 to 60-80 degrees Celsius, then add polyethylene glycol 1000 and polysorbate 40 to it, stir, cool to 40-45 degrees Celsius after dissolving, and then pour in Add the bexarotene and mix well. Keep the mixed medicinal liquid at 30-35 degrees Celsius, and then press it with the glue (composed of gelatin, glycerin, sorbitol, water and titanium dioxide) on a soft capsule machine, and obtain bexarotene soft capsules after drying, polishing and packaging . The production process data are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com