A kind of small interfering nucleic acid and pharmaceutical composition and application thereof
A composition and drug technology, applied in the field of biomedicine, can solve the problems of high frequency of administration, large amount of administration, high frequency of administration, etc., and achieve the effects of prolonging the interval of administration, reducing the amount of administration, and high biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0047] Among them, as a particularly preferred embodiment of the present invention, in the pharmaceutical composition, the molar ratio between the organic amine, cholesterol and the pegylated phospholipid is (50-70): (20 -40): (3-20).
[0048] The liposome particle formed by the siRNA of the present invention and the above-mentioned carrier has an average diameter of about 30 nm to about 200 nm, usually about 40 nm to about 135 nm, more usually, the average diameter of the liposome particle is about 50 nm to about 120 nm, From about 50 nm to about 100 nm, from about 60 nm to about 90 nm, or from about 70 nm to about 90 nm, for example, the liposome particles have an average diameter of about 30, 40, 50, 60, 70, 75, 80, 85, 90, 100, 110, 120, 130, 140, 150 or 160nm.
[0049] In a pharmaceutical composition in the form of a liposome formulation, the weight ratio (weight / weight ratio) of siRNA of the present invention to total lipids (such as organic amines, helper lipids and / or...
preparation example 1
[0060] The sequence of the siRNA is shown in Table 1, and a single strand of the siRNA has a sequence represented by SEQ ID NO:2, which is identical to the corresponding RNA interference target nucleic acid (as shown in SEQ ID NO:4) in the mRNA sequence of RRM2, The other single strand has a sequence represented by SEQ ID NO: 3, and is complementary to the corresponding RNA interference target nucleic acid in the mRNA sequence of RRM2.
[0061]The oligonucleotide single strand of siRNA is chemically synthesized according to methods known in the art. During synthesis, two deoxythymine nucleotides dTdT are added to the 3' end of the single-stranded oligonucleotide. Complementary oligonucleotide single strands anneal to form double strands, and the two ends of the double strands respectively have 3' overhangs of dTdT. The sequences of the synthesized oligonucleotides are listed in Table 1.
[0062] Table 1
[0063]
Embodiment 1
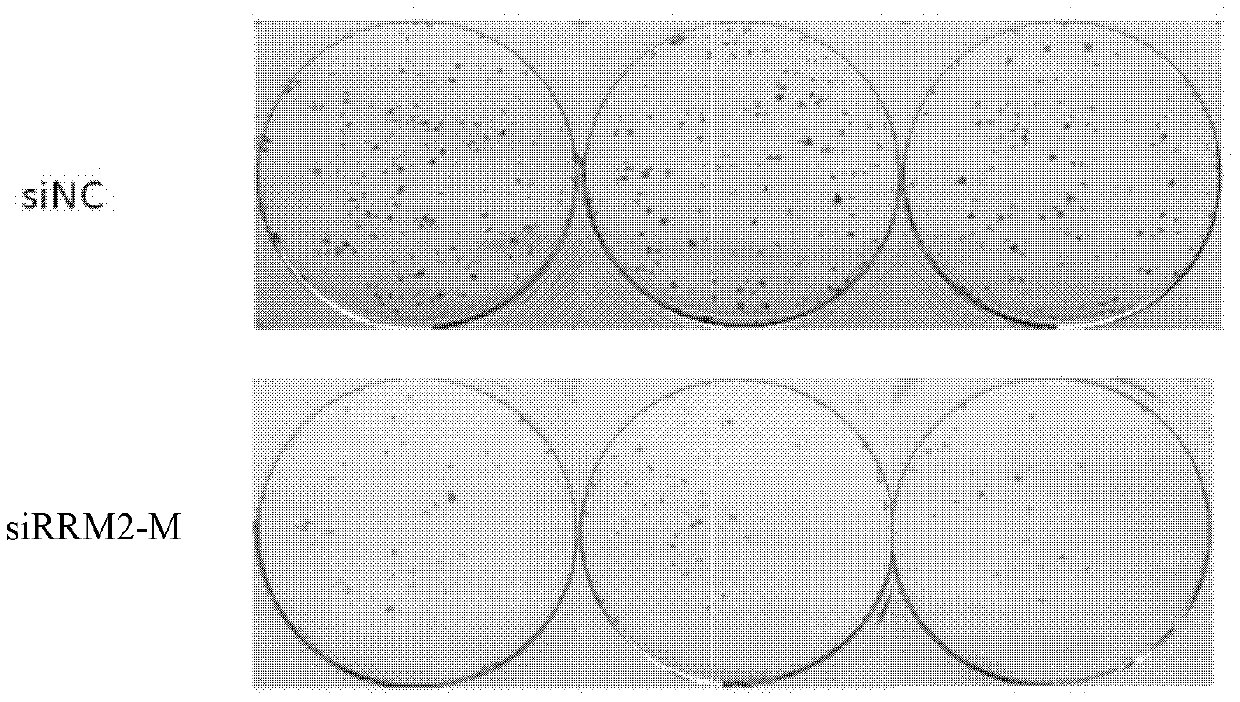
[0065] This example is used to detect the inhibitory efficiency of siRNA on the expression level of RRM2 mRNA in vitro.
[0066] The human liver cancer cell line HepG2 was inoculated in a 24-well plate with DMEM complete medium containing 10% fetal bovine serum, 2mM L-glutamine, 100U / mL penicillin, and 100g / mL streptomycin, at a cell density of 4×10 5 / well, 0.5mL per well, culture overnight at 37°C.
[0067] The specific operation steps of transfection are as follows: Dilute 100 ng of siRNA in Preparation Example 1 into 50 μL of DEME serum-free medium, and at the same time, add 1 μL of Lipofectamine TM 2000 (Invitrogen Company) was diluted in 50 μL DEME serum-free medium, and the above two solutions were incubated at room temperature for 5 minutes and then mixed. After the mixed solution was left to stand at room temperature for 20 minutes, 100 μL of the above mixed solution was added to a 24-well plate seeded with PANC-1 cells. The final concentration of siRNA was approxi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com