Method for preparing polycaprolactone polyol by using enzymatic catalysis method
A technology of polycaprolactone and enzymatic catalysis, which is applied in the field of preparation of polycaprolactone polyols and polyester polyols by enzymatic catalysis, and can solve the problem of uncontrollable content, complexity, polymer metal compound residue synthesis methods, etc. problems, to achieve the effect of improving thermodynamic properties and hydrophilic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
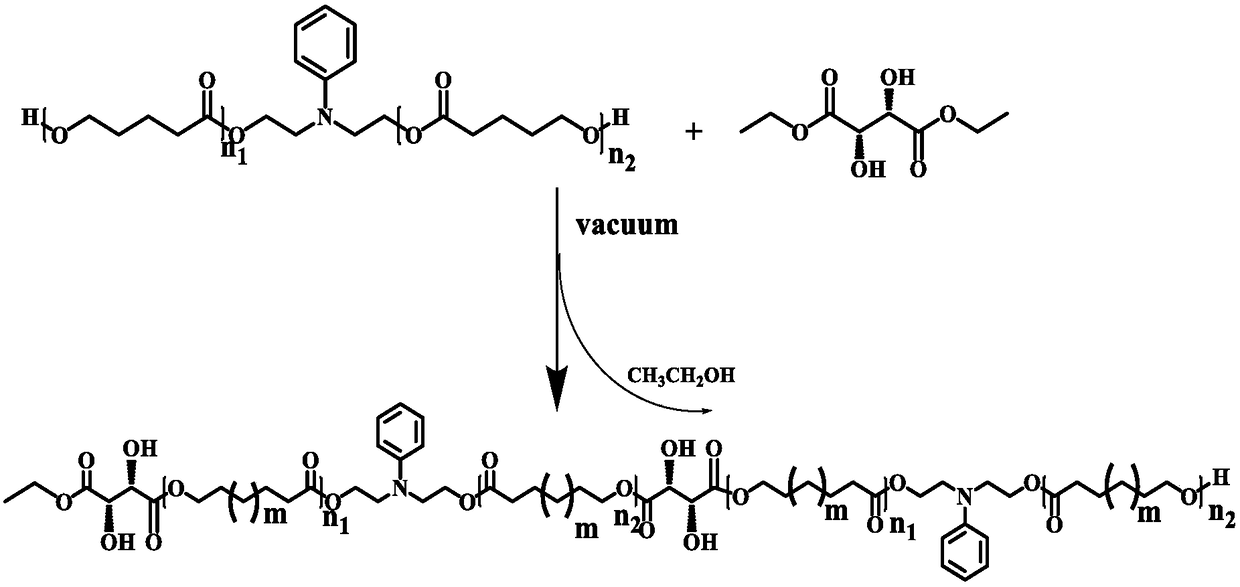
[0048] Connect a 25mL reaction flask with a magnetic stirrer to the double-row tube, and use a gas lamp to bake the tube 3 times to remove the residual moisture in the flask. Under the condition of argon gas, caprolactone monomer (1.03g, 9mmol), N-phenyldiethanolamine (1.631g, 9mmol), catalyst N435 (450mg) and solvent diphenyl ether (9.0g) were sequentially added. The argon gas was used to ventilate 3 times, and the reaction temperature was set to 70°C. After 24 hours of reaction at this temperature, the reaction can be stopped to obtain polycaprolactone prepolymer (PCL 1 ), its structural formula is shown in formula V, where n 1 +n 2 = 1.
Embodiment 2
[0050] Connect a 25mL reaction flask with a magnetic stirrer to the double-row tube, and use a gas lamp to bake the tube 3 times to remove the residual moisture in the flask. Under argon, add caprolactone monomer (3.078g, 27mmol), N-phenyldiethanolamine (0.979g, 5.4mmol), catalyst N435 (510mg) and solvent diphenyl ether (10.32g) in sequence . The argon gas was used to ventilate 3 times, and the reaction temperature was set to 70°C. After 24 hours of reaction at this temperature, the reaction can be stopped to obtain polycaprolactone prepolymer (PCL 5 ), its structural formula is shown in formula V, where n 1 +n 2 = 5.
Embodiment 3
[0052] Connect a 25mL reaction flask with a magnetic stirrer to the double-row tube, and use a gas lamp to bake the tube 3 times to remove the residual moisture in the flask. Under the condition of argon gas, caprolactone monomer (3.42g, 30mmol), N-phenyldiethanolamine (0.381g, 2mmol), catalyst N435 (430mg) and solvent diphenyl ether (8.42g) were sequentially added. The argon gas was used to ventilate 3 times, and the reaction temperature was set to 70°C. After 24 hours of reaction at this temperature, the reaction can be stopped to obtain polycaprolactone prepolymer (PCL 15 ), its structural formula is shown in formula V, where n 1 +n 2 = 15.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com