Use of Alkyl Gallates as Glyoxal and Methylglyoxal Inhibitors
A technology of alkyl gallate and methylglyoxal, which is applied in the fields of application, edible oil/fat, anti-toxic agent, etc., can solve the problem of undiscovered alkyl gallate, etc., and achieve the effect of preventing harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
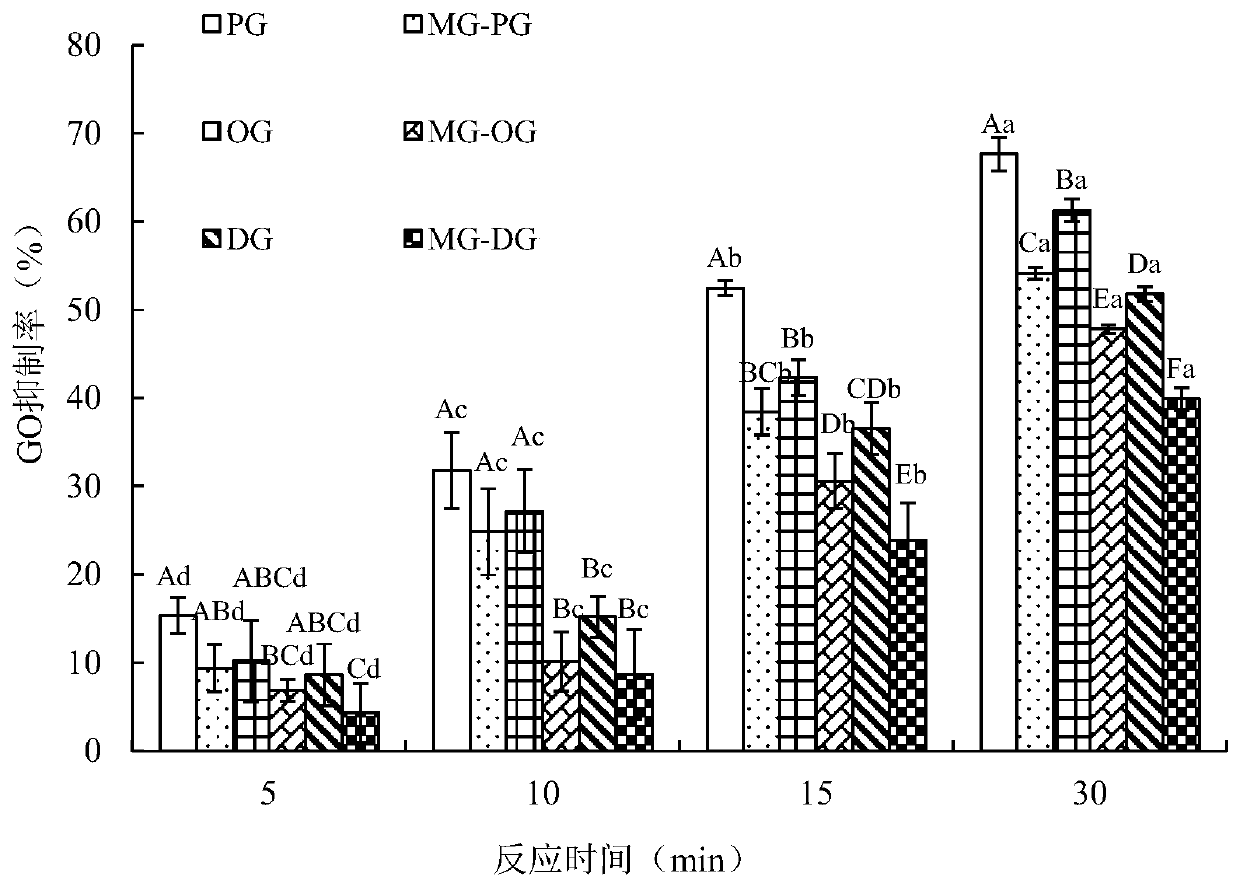
[0028] Embodiment 1PG / OG / DG and adduct product MG-PG / MG-OG / MG-DG are to the inhibitory activity of GO
[0029] 1) Experimental materials and instruments
[0030] MG-PG (the adduct product of PG and GO, HPLC, ≥90%), MG-OG (the adduct product of OG and GO, HPLC, ≥90%), (the adduct product of DG and GO, MG -DG, HPLC, ≥90%) are all self-made in the laboratory.
[0031] PG, OG, and DG were all purchased from Shanghai Sinopharm Chemical Reagent Co., Ltd.; GO (40% aqueous solution) was purchased from Sigma-Aldrich Company of the United States; 7820A gas chromatography (Agilent Company of the United States).
[0032] 2) Experimental steps
[0033]Add 2 mL of PG (or OG, DG, MG-PG, MG-OG, MG-DG) methanol solution with a concentration of 1 mmol / L, 2 mL of PBS with a concentration of 1 mmol / L (0.2 mol / L, pH7. 0) The prepared GO solution was vortex mixed, reacted in an oil bath at 100°C for 5, 10, 15, and 30 minutes, and then quickly took it out and placed it in an ice-water bath to coo...
Embodiment 2M
[0036] Example 2 Purification and structural research of MG-PG, MG-OG, MG-DG, MM-PG
[0037] 1) Experimental materials and instruments
[0038] AVANCE 400MHz nuclear magnetic resonance instrument (Bruker); 1290 / 6460 liquid chromatography-mass spectrometry (Agilent, USA).
[0039] 2) Experimental steps
[0040] The molar ratio of PG to GO is 1:10 for the reaction, and the C 18 The chromatographic column is purified to obtain MG-PG (MG-OG, MG-DG, MM-PG are prepared in the same way), and the molecular weight is analyzed by LC-MS; 1D-NMR 1 H, 13 C, 2D-NMR 1 H- 13 C HMQC and HMBC for structural analysis. The structural analysis of MG-OG, MG-DG, and MM-PG is the same as above.
[0041] 3) Experimental results
[0042] The prepared MG-PG was determined by liquid chromatography-mass spectrometry, and in negative ion mode, m / z was 269[M-H] - , than m / z211[M-H] of PG - More than 58 (MW GO is 58), and 269[M-H] in MS / MS - The main fragment ion peak is m / z 211, missing a GO mol...
Embodiment 3
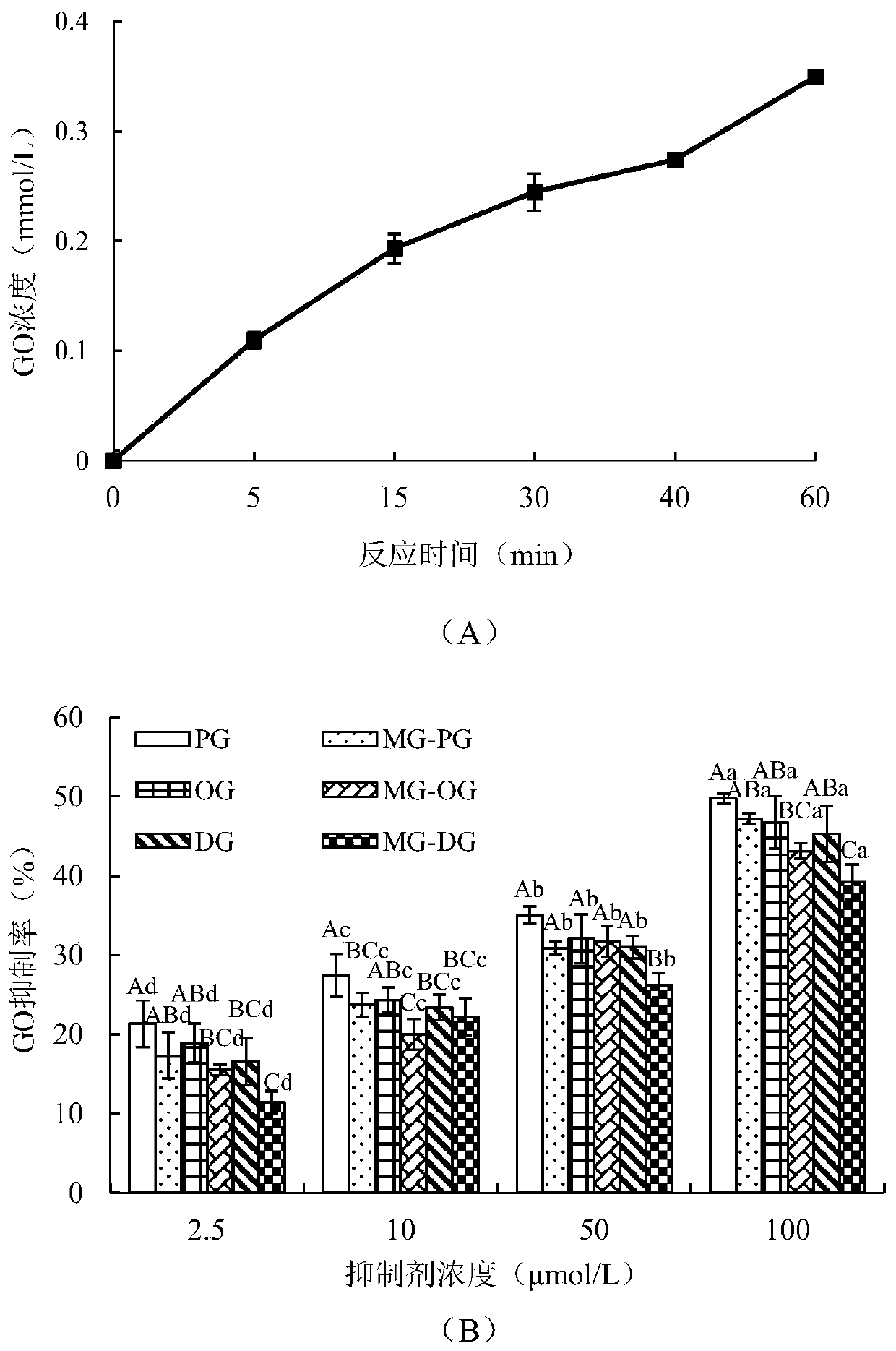
[0047] Example 3 Determination of PG / OG / DG and the addition product MG-PG / MG-OG / MG-DG inhibiting the formation of GO in the amino acid-sugar system under high temperature conditions
[0048] 1) Experimental materials and instruments
[0049] Arginine (Arg, analytically pure) and glucose (analytical pure) were purchased from Shanghai Biological Biotechnology Co., Ltd.; 7820A gas chromatograph (Agilent, USA).
[0050] 2) Experimental steps
[0051] Add 2 mL of Arg solution and glucose solution prepared in PBS (0.2 mol / L, pH 7.0) with a concentration of 180 mmol / L to the explosion-proof vial in turn, and dilute the PBS to a final volume of 6 mL. After vortex mixing, place in an oil bath at 170°C, react for 0, 5, 15, 30, and 40 minutes respectively, then quickly take it out and place it in an ice-water bath to cool. A 1 mL sample was taken for derivatization treatment, and the GO content was detected by gas chromatography. Three sets of parallels were made for each sample.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com