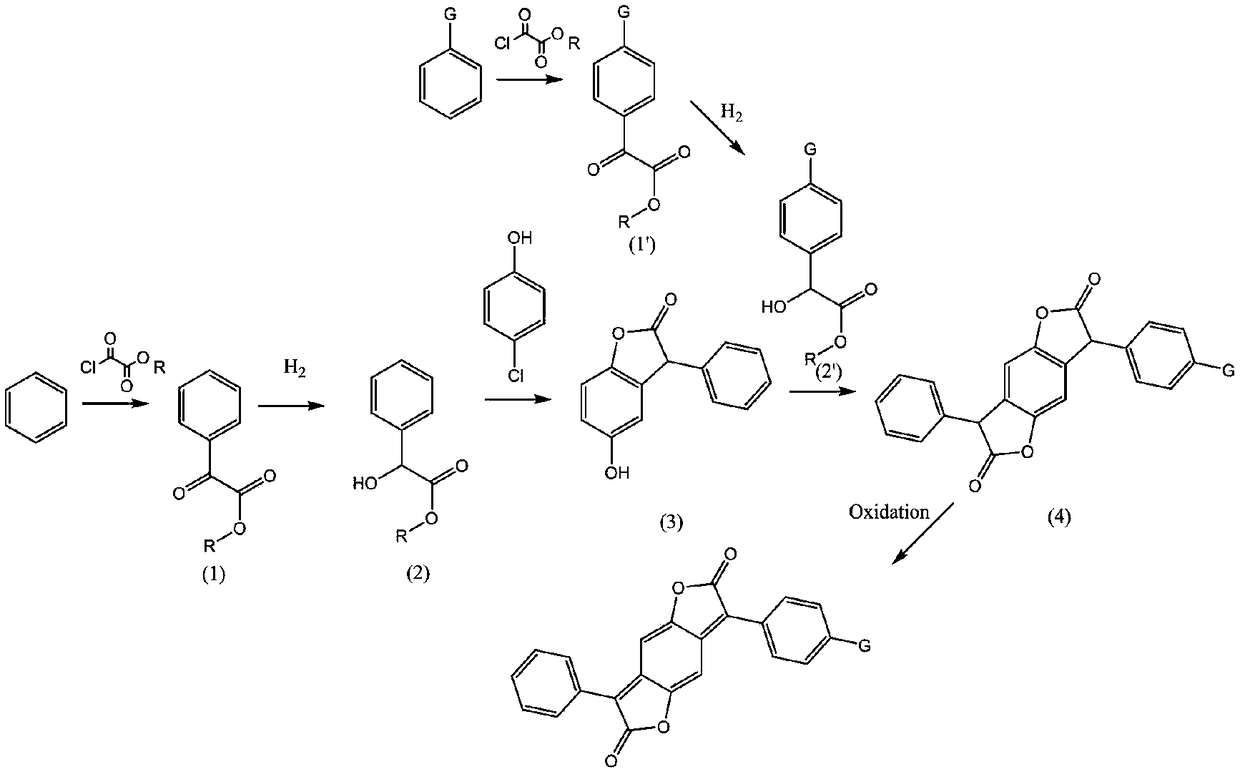
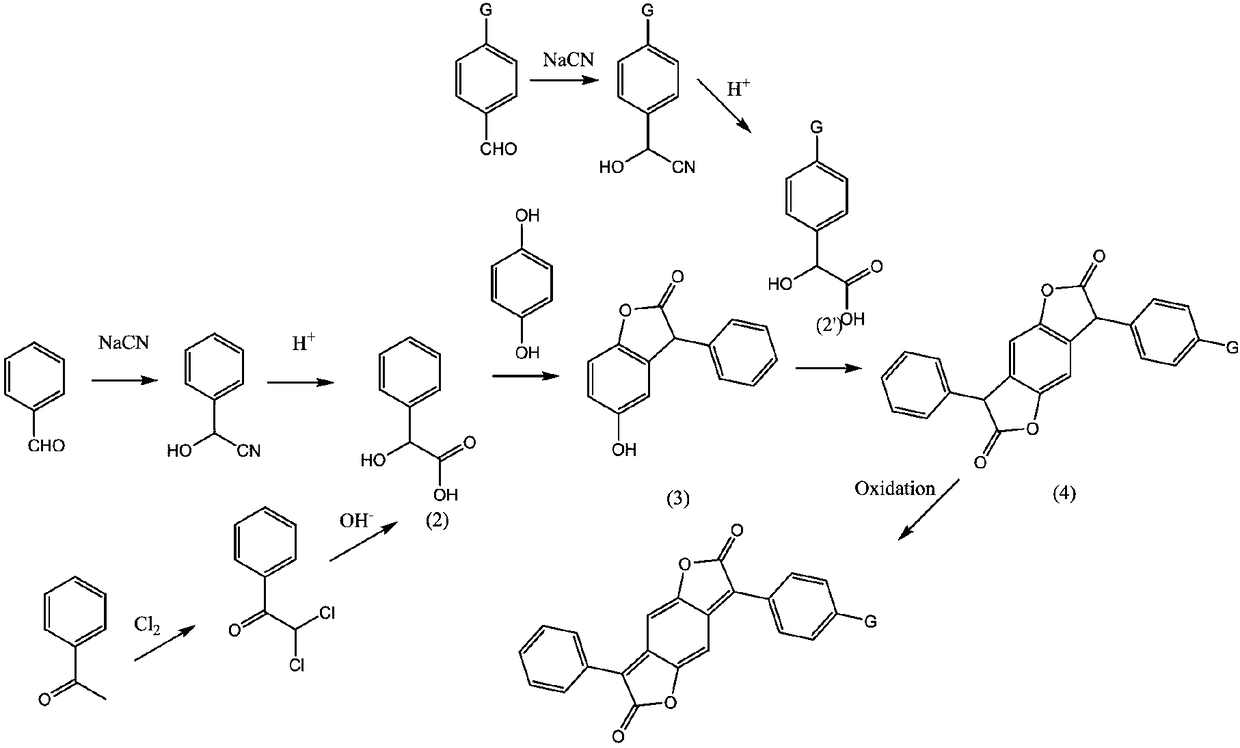
Benzodifuranone dye synthetic method
The technology of a kind of benzodifuranone and the synthetic method is applied in the field of chemical industry, which can solve the problems of high production cost of disperse dyes, unavoidable highly toxic sodium cyanide, dangerous production process, etc., and achieves cheap and easy-to-obtain production raw materials, good industrial Application prospect, low risk effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Add monomethyl oxalyl chloride (73.4g, 599mmol) to a solution of aluminum trichloride (92.5g, 693mmol) in dichloromethane (800g) at 0-5°C, and stir for 30 minutes at this temperature Benzene (39g, 500mmol) was added dropwise in the reaction solution, after the dropwise addition, the reaction solution was stirred at room temperature for 4 hours, the reaction solution was poured into the ice-water mixture, the organic layer was separated, and the aqueous layer was extracted with dichloromethane. The organic layers were combined, and the solvent was recovered in vacuo, and the inside of the kettle was a light yellow viscous liquid (Intermediate 1) (82g, yield 94%);
[0030] (2) Add monomethyl oxalyl chloride (73.4g, 599mmol) to a solution of aluminum trichloride (92.5g, 693mmol) in dichloromethane (800g) at 0-5°C, and stir for 30 minutes at this temperature ; In the reaction solution, dropwise add the dichloromethane (120g) solution of phenbutyl ether (75g, 500mmol), d...
Embodiment 2
[0036] (1) Add monoethyl oxalyl chloride (81.5g, 599mmol) to a solution of zinc dichloride (95.2g, 700mmol) in chloroform (800g) at 0-5°C, and stir for 30 minutes at this temperature; Benzene (39g, 500mmol) was added dropwise to the reaction solution. After the addition was complete, the reaction solution was stirred at room temperature for 4 hours. The reaction solution was poured into a mixture of ice and water, and the organic layer was separated. The aqueous layer was extracted with chloroform, and the organic layers were combined. The solvent was recovered in vacuo, and the still was light yellow viscous liquid (intermediate 1) (89g, yield 95%);
[0037](2) Add monoethyl oxalyl chloride (81.5g, 599mmol) to a solution of zinc dichloride (95.2g, 700mmol) in chloroform (600g) at 0-5°C, and stir for 30 minutes at this temperature; A solution of 2-(phenoxymethyl)tetrahydrofuran (106.6 g, 599 mmol) in chloroform (120 g) was added dropwise to the reaction solution. After the add...
Embodiment 3
[0043] (1) Add monomethyl oxalyl chloride (73.4g, 599mmol) to a solution of aluminum trichloride (95.2g, 700mmol) in ethylene dichloride (800g) at 0-5°C, and stir for 30 minutes; in the reaction solution, benzene (39g, 500mmol) was added dropwise, after the dropwise addition, the reaction solution was stirred at room temperature for 4 hours, the reaction solution was poured into the ice-water mixture, the organic layer was separated, and the aqueous layer was extracted with dichloromethane , merging the organic layers, and recovering the solvent in vacuo, the still was light yellow viscous liquid (intermediate 1) (83g, yield 95%);
[0044] (2) Add monomethyl oxalyl chloride (73.4g, 599mmol) to a solution of ferric chloride (113.7g, 700mmol) in ethylene dichloride (600g) at 0-5°C, and stir for 30 minute; in the reaction solution, dropwise add the dichloromethane (120g) solution of phenylpropyl ether (81.5g, 599mmol), dropwise, the reaction solution was stirred at room temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com