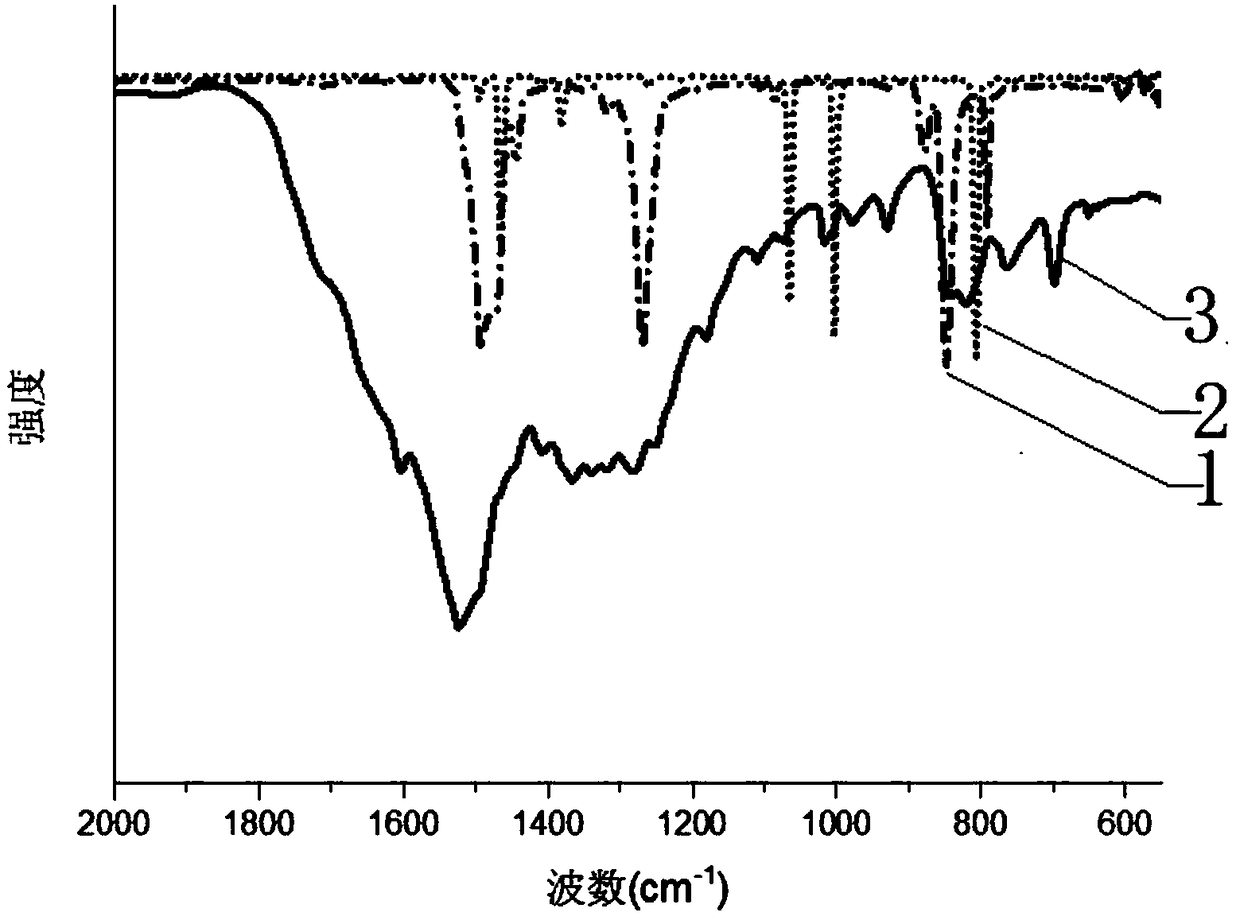
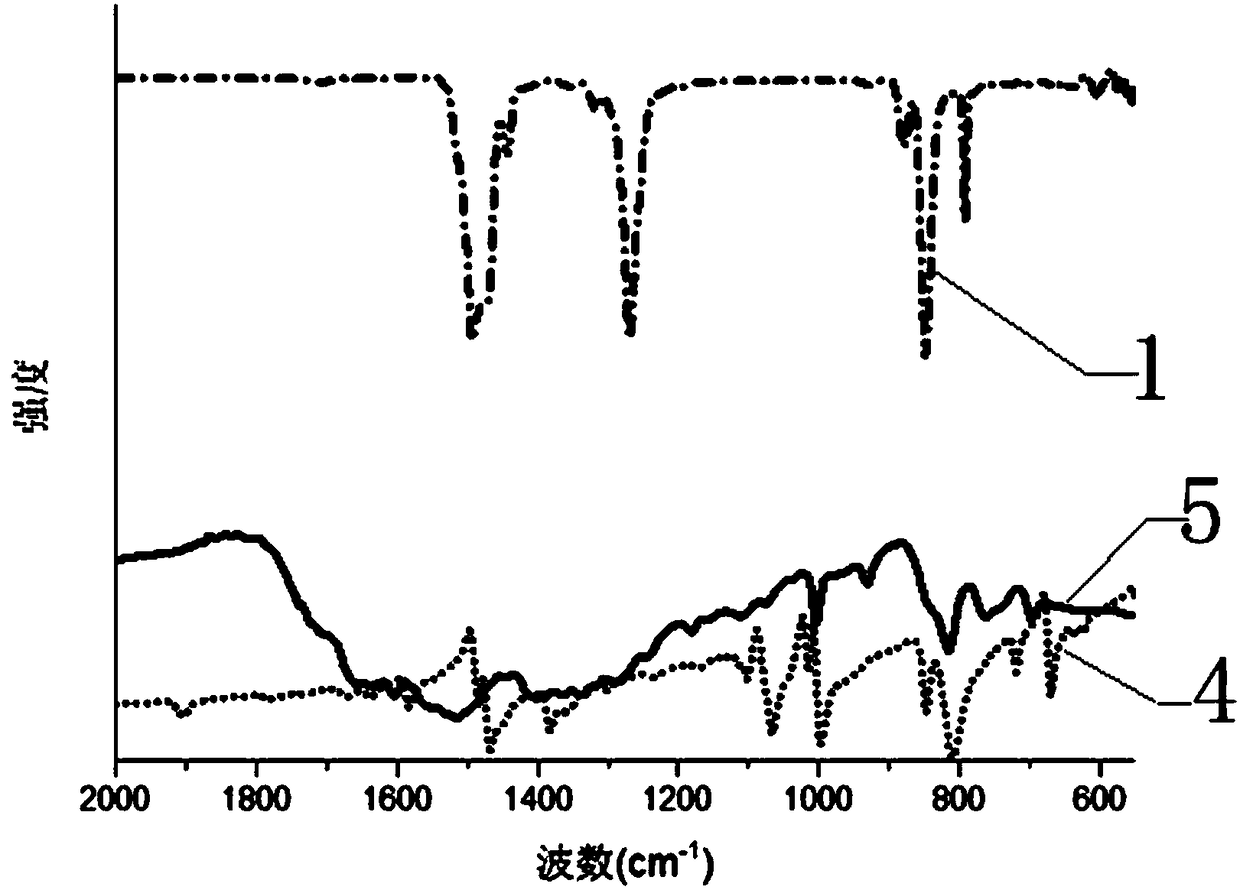
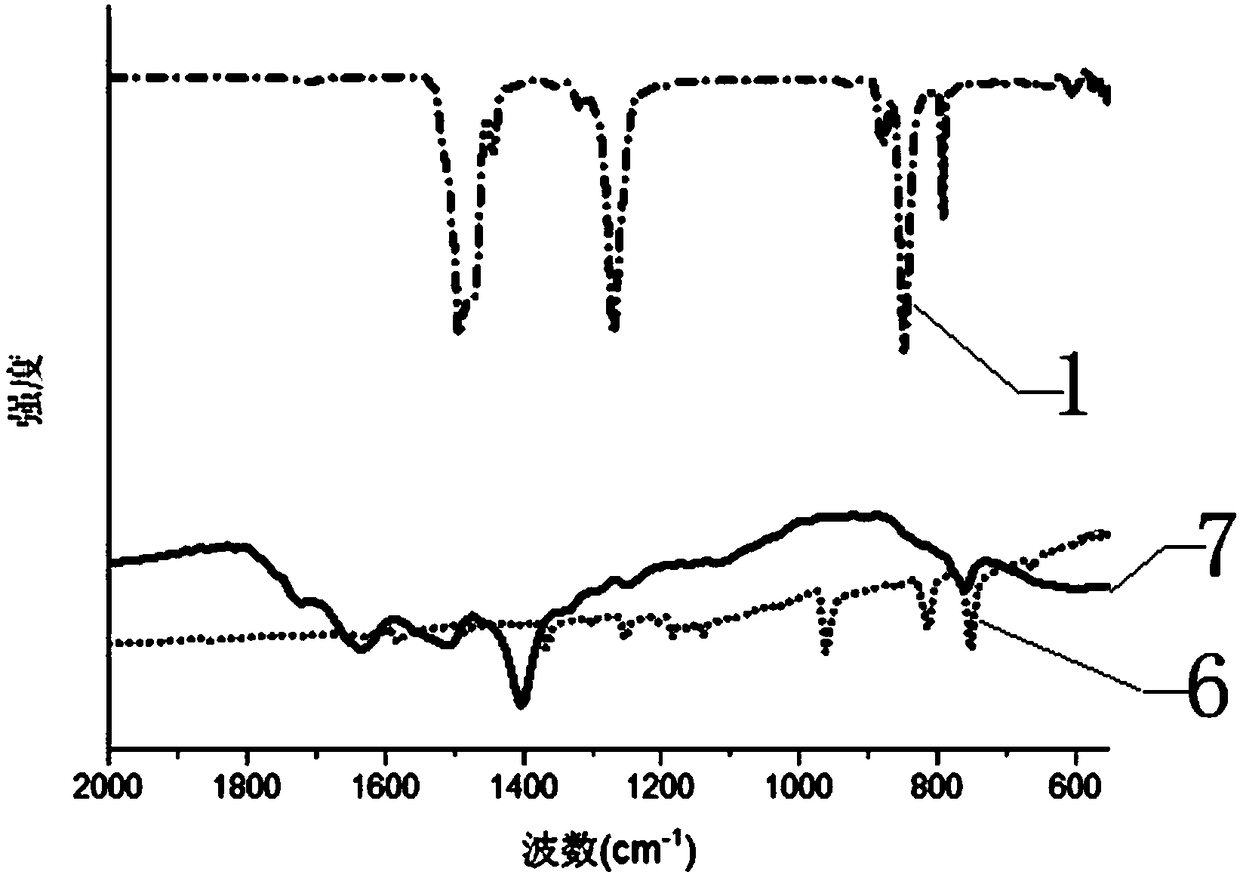
Large-scale preparation method for covalence copolymer with triazine frame
A technology for large-scale preparation of triazine compounds, which is applied in the field of large-scale preparation of covalent polymers with triazine skeletons, can solve the problems of harsh reaction vessel requirements, difficulty in large-scale preparation, and carbonization of products, and achieves low production cost, Easy to industrialize and scale up production, easy to achieve effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Accurately weigh 1,4-dibromobenzene (30mmol, 3 equivalents, 7070mg, purity: 98%) and dissolve it in toluene in a 20mL three-necked flask. The solution (61mmol, 6.1 equivalents, 24.4mL, 2.5mol / L) was added dropwise into a three-necked flask, and at the same time, toluene was added, stirred for 30min, and heated to reflux to prepare 1,4-dilithiumbenzene (nucleophile).
[0067] 2,4,6-Trichloro-1,3,5-triazine (20mmol, 2eq, 3763mg, purity: 98%) was dissolved in 10mL of dry 1,4-dioxane, and then the dissolved 2 , 4,6-trichloro-1,3,5-triazine was slowly dropped into the three-necked flask, and refluxed for 22 hours.
[0068] After reflux, cool to room temperature naturally, add saturated ammonium chloride solution to quench the reaction, filter the filter residue, wash the filter residue with water to remove lithium salt, then wash with ethyl acetate, methanol and other organic solvents to remove small molecule products in the filter residue, and finally The filter residue wa...
Embodiment 2
[0071] Accurately weigh 4,4'-dibromobiphenyl (15mmol, 3 equivalents, 4775mg, purity: 98%) and dissolve it in toluene in a 20mL three-necked flask, and replace the air with nitrogen as a protective gas. The n-hexane solution (30.5mmol, 6.1 equivalents, 12.2mL, 2.5mol / L) was added dropwise into a three-necked flask, and at the same time, toluene was added, stirred for 30min, and heated to reflux to prepare 4,4'-dilithium biphenyl (nucleophilic Reagent).
[0072] 2,4,6-Trichloro-1,3,5-triazine (10 mmol, 2 equivalents, 1882 mg, purity: 98%) was dissolved in 5 mL of dry 1,4-dioxane, and the dissolved 2 , 4,6-trichloro-1,3,5-triazine was slowly added dropwise into the three-necked flask, and refluxed for 22 hours.
[0073] After reflux, cool to room temperature naturally, add saturated ammonium chloride solution to quench the reaction, filter the filter residue, wash the filter residue with water to remove lithium salt, then wash with ethyl acetate, methanol and other organic solve...
Embodiment 3
[0076] Accurately weigh 1,4-dibromonaphthalene (15mmol, 3 equivalents, 4377mg, purity: 98%) and dissolve it in toluene in a 20mL three-necked flask, and replace the air with nitrogen as a protective gas. Add alkane solution (30.5mmol, 6.1 equivalents, 12.2mL, 2.5mol / L) dropwise into a three-necked flask, add toluene at the same time, stir for 30min, and heat to reflux to prepare 1,4-dilithium naphthalene (nucleophile).
[0077] 2,4,6-Trichloro-1,3,5-triazine (10 mmol, 2 equivalents, 1882 mg, purity: 98%) was dissolved in 5 mL of dry 1,4-dioxane, and the dissolved 2 , 4,6-trichloro-1,3,5-triazine was slowly added dropwise into the three-necked flask, and refluxed for 22 hours.
[0078] After reflux, cool to room temperature naturally, add saturated ammonium chloride solution to quench the reaction, filter the filter residue, wash the filter residue with water to remove lithium salt, then wash with ethyl acetate, methanol and other organic solvents to remove small molecule produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com