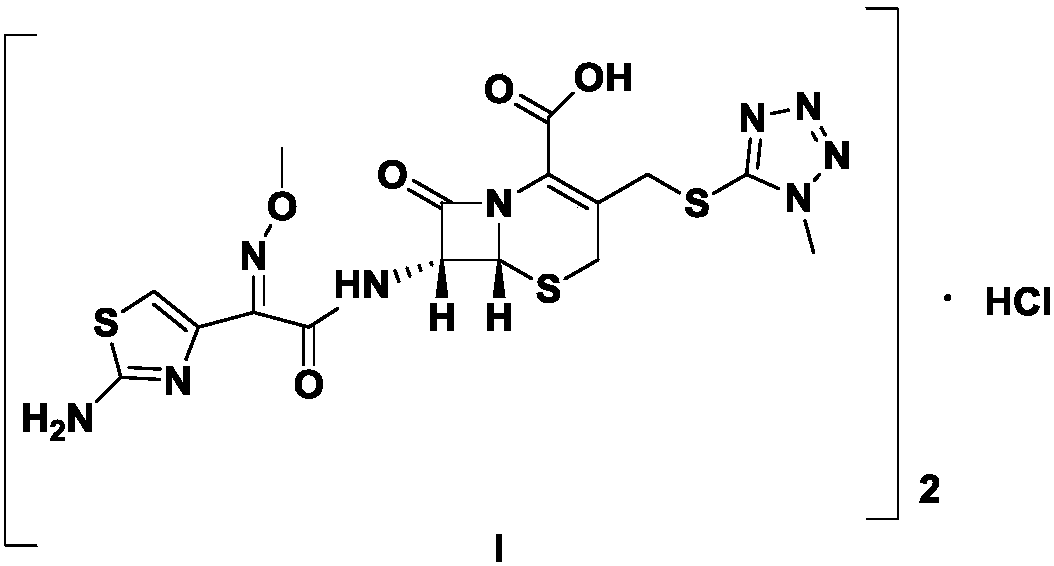
Preparation method of cefmenoxime hydrochloride
A technology of cefmenoxime hydrochloride and hydrochloride, which is applied in the field of medicine and chemical industry, can solve the problems of high cost, low product purity and yield, and increased cost, and achieve the effects of less pollution, improved color, and reduced degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
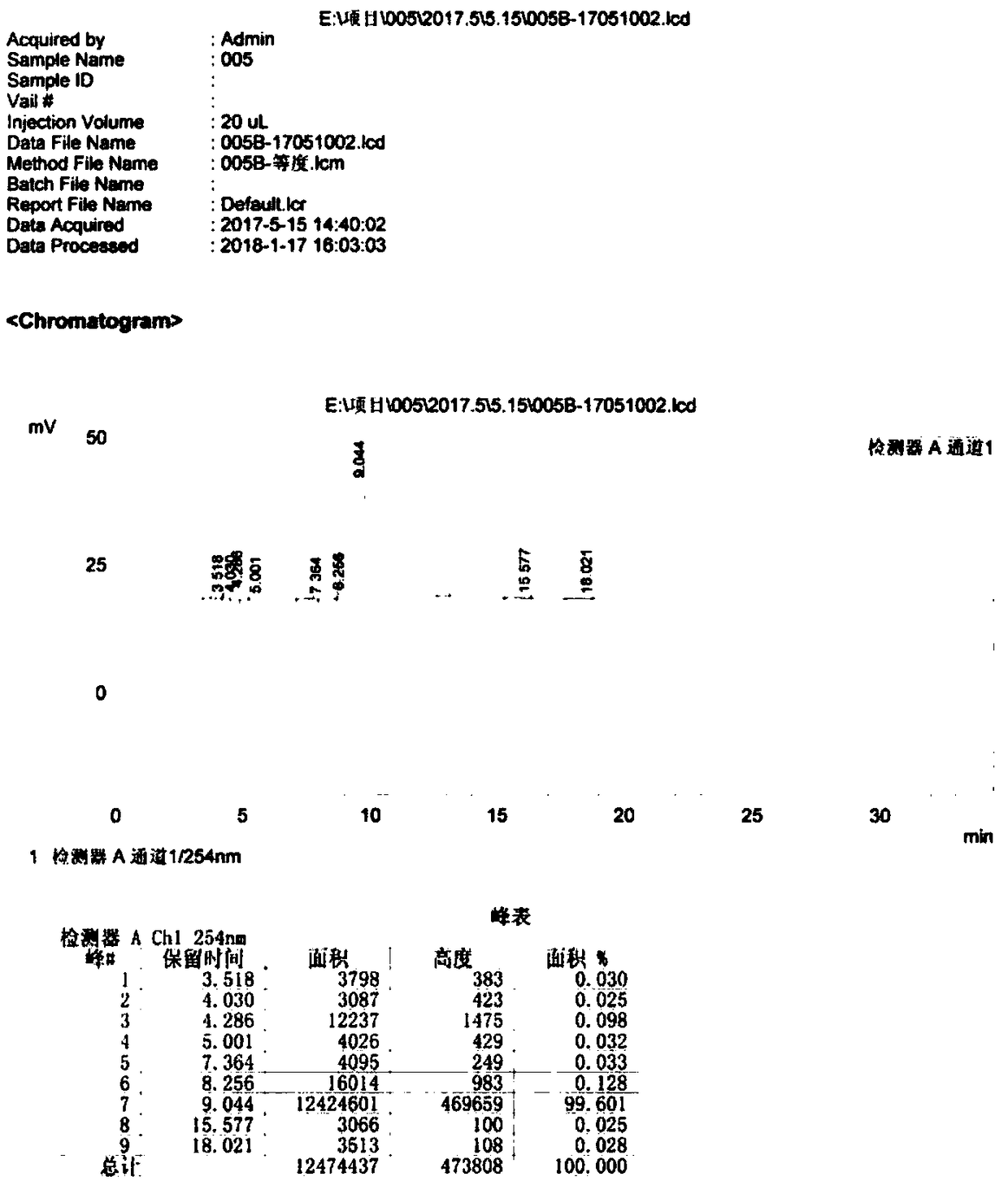
Image
Examples
Embodiment 1
[0044] (1) Under stirring, add 100.0g (0.5mol) of aminothiaxamic acid to 1000ml of dichloromethane, then add 106.0g (0.52mol) of N,O-bis(trimethylsilyl)acetamide dropwise, and add it in about 0.5h. , Then heat to 40℃;
[0045] (2) Add 227.7g (2.25mol) of triethylamine dropwise to the solution of the compound of formula III, dropwise add 62.0g (0.52mol) of thionyl chloride, add it in about 0.5h, control the temperature at 0℃ and react for 2h, then cool to At -17~-13°C, add 174.2g (0.47mol) of 7-ATCA.HCl, react at this temperature for 2h, and monitor the reaction with 7-ATCA.HCl≤1.0%. After the reaction is completed, add 1000 ml of water, adjust the pH value to 6-7 with 35% hydrochloric acid solution, stir to separate the liquids, and retain the aqueous phase; the organic phase is extracted with 500 ml and 300 ml of water successively, and the aqueous phases are combined.
[0046] (3) Add 25 g of activated carbon and 25 g of alumina to the combined water phase to decolorize for 0.5 ...
Embodiment 2
[0048] (1) Add 100.0g (0.5mol) of axamic acid to 1000ml of dichloromethane while stirring, then add 84.0g (0.52mol) of hexamethyldisilazane dropwise, add it in about 0.5h, and then heat to 45°C, After stirring for 4 hours, it was cooled to 2°C, and the solution of the compound of formula III was directly used in the next reaction.
[0049] (2) Add 258.5g (2.0mol) of N,N-diisopropylethylamine, 62.7g (0.52mol) of pivaloyl chloride dropwise to the solution of the compound of formula III, add it in about 0.5h, and control the temperature at 2℃ React for 3h, then cool to -19~-15°C, add 166.0g (0.45mol) of 7-ATCA.HCl, react at this temperature for 3h, reaction monitoring 7-ATCA.HCl≤1.0%. After the reaction is completed, add 1000 ml of water, adjust the pH to 6-7 with 35% hydrochloric acid solution, stir and separate the liquids, and retain the aqueous phase; the organic phase is extracted with 500 ml and 400 ml of water in turn, and the aqueous phases are combined.
[0050] (3) Add 12.5...
Embodiment 3
[0052] (1) Under stirring, add 100.0g (0.5mol) of the axamic acid of formula II into a mixed solvent of 900ml of dichloromethane and 100ml of N,N-dimethylformamide (DMF), and then add dropwise hexamethyldisilazane After adding 82.3g (0.51mol) in about 0.5h, it was heated to 42°C, stirred for 2h, and then cooled to 2°C. The solution of the compound of formula III was directly used in the next reaction.
[0053] (2) Add 258.5g (2.0mol) of N,N-diisopropylethylamine dropwise to the solution of the compound of formula III, add 59.5g (0.50mol) of thionyl chloride dropwise, and finish adding in about 0.5h, control the temperature to 0 React at ℃ for 2h, then cool to -17~-13℃, add 174.2g (0.47mol) of 7-ATCA.HCl, react at this temperature for 2h, and monitor the reaction with 7-ATCA.HCl≤1.0%. After the reaction is completed, add 1000 ml of water, adjust the pH value to 6-7 with 35% hydrochloric acid solution, stir to separate the liquids, and retain the aqueous phase; the organic phase is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com