Composite anti-restenosis drug and its controlled release system of coronary drug-eluting stent
A technology for eluting stents and restenosis, applied in drug combination, drug delivery, cardiovascular system diseases, etc., can solve problems such as thrombosis, reduce the incidence of thrombus, prevent thrombosis, and have excellent anti-restenosis effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. In vitro experiment on the effect of arsenic trioxide on porcine coronary vascular smooth muscle cells and endothelial cells
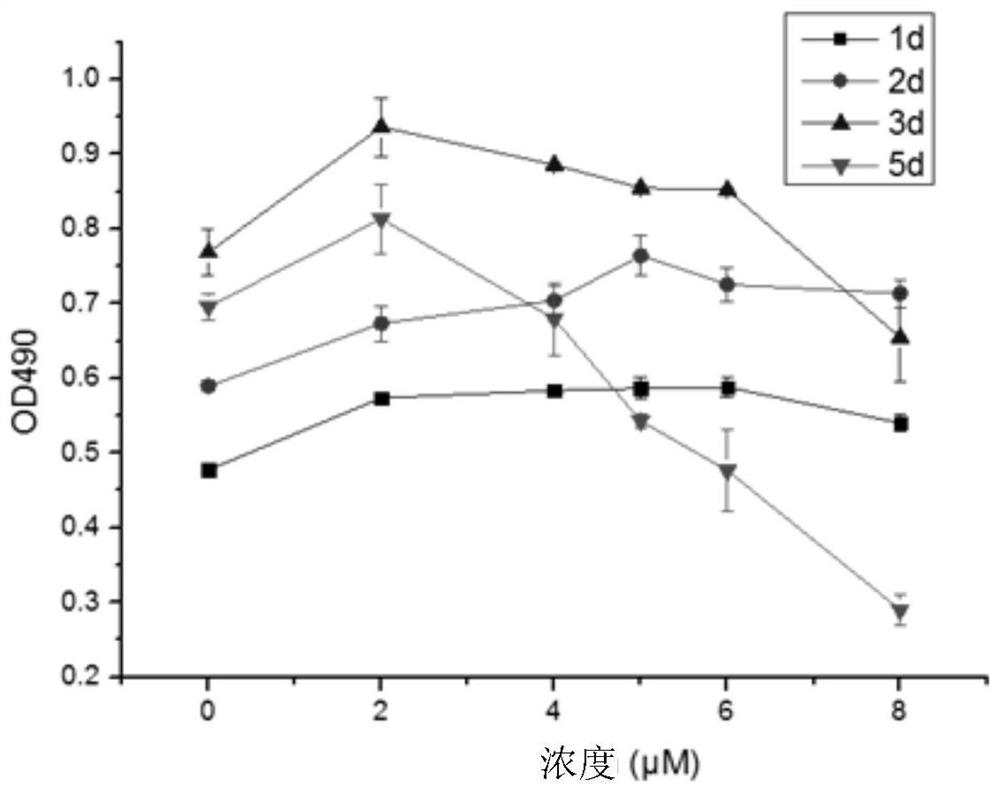
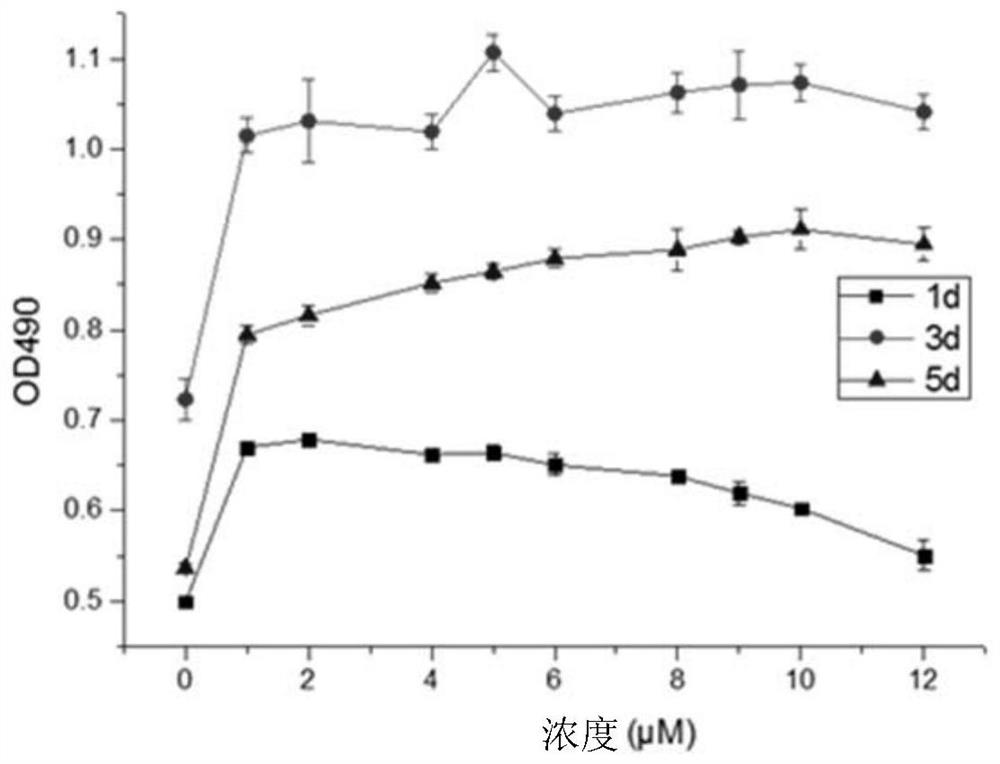
[0043] figure 1 with figure 2 It shows the effect of arsenic trioxide on porcine coronary vascular smooth muscle primary cells and endothelial primary cells in vitro. The vertical axis in the figure represents the total number of cells in the wells of the culture plate, and the horizontal axis represents the concentration of the arsenic trioxide solution. Each curve represents the effect of different concentrations of arsenic trioxide on cell apoptosis (decrease in the total number of cells) at a certain time. From the figure ( figure 2 ) It can be clearly seen that the effect of arsenic trioxide on endothelial cells (within the concentration of 12 μM) is weak or even promotes the proliferation of endothelial cells to a certain extent ( figure 2 ). But for smooth muscle cells from the 2nd day, even at a low concentration (3μM), the ce...
Embodiment 2
[0049] In this example, two implementation methods are listed to realize the controlled release of the drug by changing the structure (single layer or multi-layer) of the drug release system (drug coating) and the ratio of the drug and the polymer material in each layer .
Embodiment approach 1
[0050] Implementation method 1: composite drug monolayer structure release system
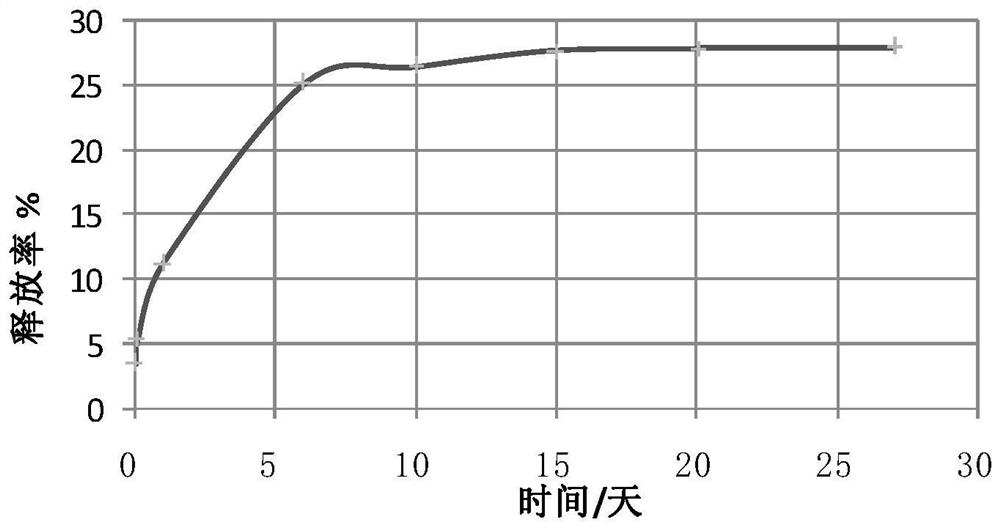
[0051] The drug-mixed single-layer structure release system contains arsenic trioxide, rapamycin and polymer material PLGA. By adjusting the ratio between drugs and between drugs and polymer materials, the shape of the drug release curve can be changed to achieve the purpose of controlled drug release.
[0052] As long as there is no harmful interaction between the mixed drugs, in theory, multiple anti-restenotic drugs such as arsenic trioxide, rapamycin or paclitaxel can be selected for drug mixing. In consideration of clinical and manufacturing optimization, two drugs, arsenic trioxide and rapamycin, can be selected to be mixed. For further optimization considerations, in the formulation of the mixed drug, the dosage of arsenic trioxide is 4-8 μg / mm, and the dosage of rapamycin is 1-5 μg / mm. This amount is used to open a stent with an outer diameter of 3.0 mm, and the amount of drug used fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com