A kind of SGRNA and the chronic virus carrier and application they built
A lentiviral vector, recombinant lentivirus technology, applied in lentiviral vector, clinical treatment application of human liver tumor patients, and liver arterial embolization field, can solve problems such as obvious treatment defects, and achieve great potential for clinical use, obvious effect, To achieve effective treatment and life extension effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
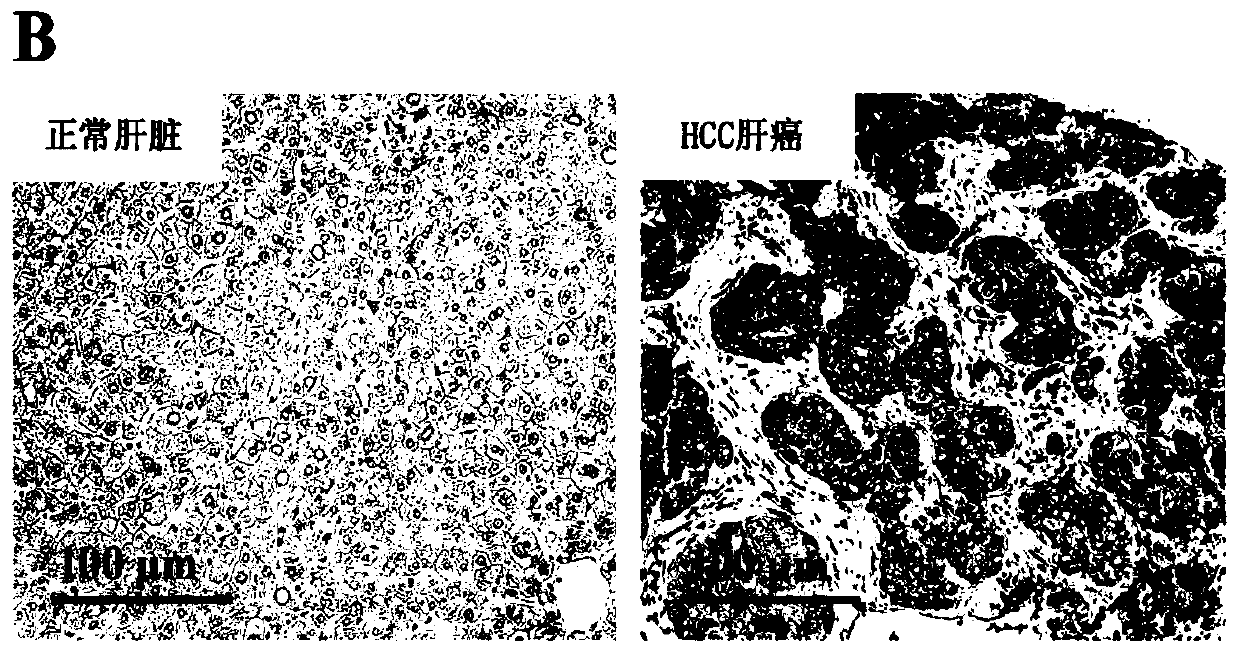
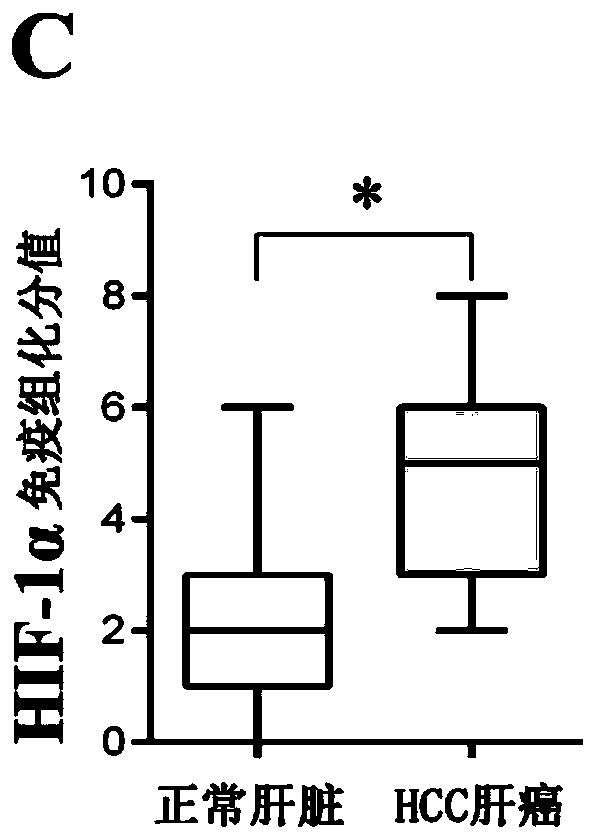
[0044] Example 1: Detection of the expression of HIF1-α in HCC tumor tissue
[0045] The method for detecting the expression of HIF1-α in HCC tumor tissue comprises the steps of:
[0046] 1. Western blot detection of HIF1-α expression in HCC tumor tissue
[0047] (1) Sample preparation: 1) Hepatobiliary and pancreatic surgery in Shenzhen People's Hospital took tumors and paracancerous tissues from patients with HCC liver cancer, and stored them in liquid nitrogen; 2) Added liquid nitrogen and grinded quickly to obtain tumor and normal tissue powder, RIPA lysate Lyse the sample; 3) Centrifuge at 12000g / min at 4°C for 20 minutes; 4) Transfer the supernatant to a new tube, measure the protein concentration with a protein quantification kit, and record it, add 1 / 5 volume of 5× The loading buffer was mixed with a gun and boiled in a boiling water bath for 5 minutes; 5) Centrifuged at the maximum speed of 4°C for 10 minutes; 6) The supernatant was transferred to a new tube and ali...
Embodiment 2
[0074] Example 2: Construction of CRISPR / Cas9 lentivirus targeting HIF1-α gene knockout
[0075] The method for constructing the CRISPR / Cas9 lentivirus comprises the steps:
[0076] 1. Construction of pLenti-CAS-sg719 / sg720 / sg721-egfp plasmid
[0077] The target sgRNAs (including sgRNA719, sgRNA720 and sgRNA721) targeting the first exon sequence of HIF-1α were designed by online software (www.crispr.mit.edu). The nucleotide sequences of the three sgRNAs designed are as follows:
[0078] sgRNA719 (SEQ ID NO.1): CCTCACACGCAAATAGCTGA;
[0079] sgRNA720 (SEQ ID NO.2): TACTCATCCATGTGACCATG;
[0080] sgRNA721 (SEQ ID NO.3): GTTATGGTTCTCACAGATGA;
[0081] The target sgRNAs were inserted into the plasmid pLenti-CAS-sgRNA-egfp (GeneChem, Shanghai) and identified by sequencing, and pLenti-CAS-sg719 / sg720 / sg721-egfp was successfully constructed, as shown in Figure 2(A);
[0082] As shown in Figure 2(A), we designed three different sgRNAs targeting the first exon sequence of HIF-1α,...
Embodiment 3
[0098] Example 3: Knockout of SMMC-7721 cells and HCC tumor HIF1-α gene by lentivirus expressing CRISPR / Cas9
[0099] 1. Infection of SMMC-7721 cells with lentivirus expressing CRISPR / Cas9
[0100] (1) will 3×10 5 A SMMC-7721 cell (ATCC, USA) was plated in a 6-well plate, and two cell plates were plated in total. The cells were cultured in DMEM (GIBCO) with complete medium, including 10% FBS (GIBCO);
[0101] (2) After culturing for 8 hours, add LV-Ctrl, LV-H719, LV-H720 and LV-H721 to the supernatant culture solution, add Polybrene (Sigma), and repeat 3 times for each sample;
[0102] (3) After 6-8 hours, remove the supernatant medium and add fresh medium; in the hypoxia experiment, 150 μM CoCl should be added to the medium 2 (Sigma) as a hypoxia inducer;
[0103] (4) After 48 hours, collect the cells and take a part of the flow cytometry (C6, BD Biosciences) to detect the expression rate of GFP positive cells;
[0104] (5) Use the QIAamp DNA blood kit (QIAGEN) to extra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com