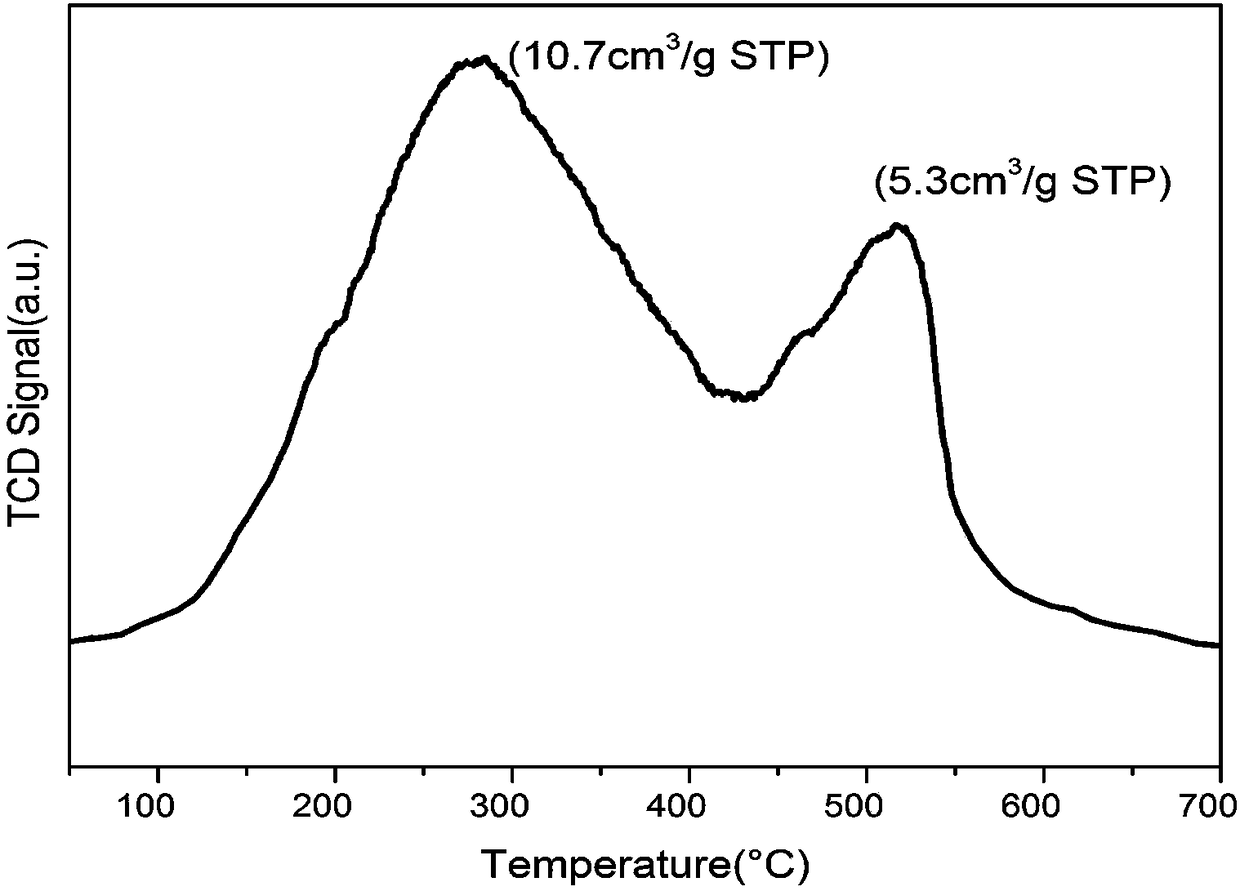
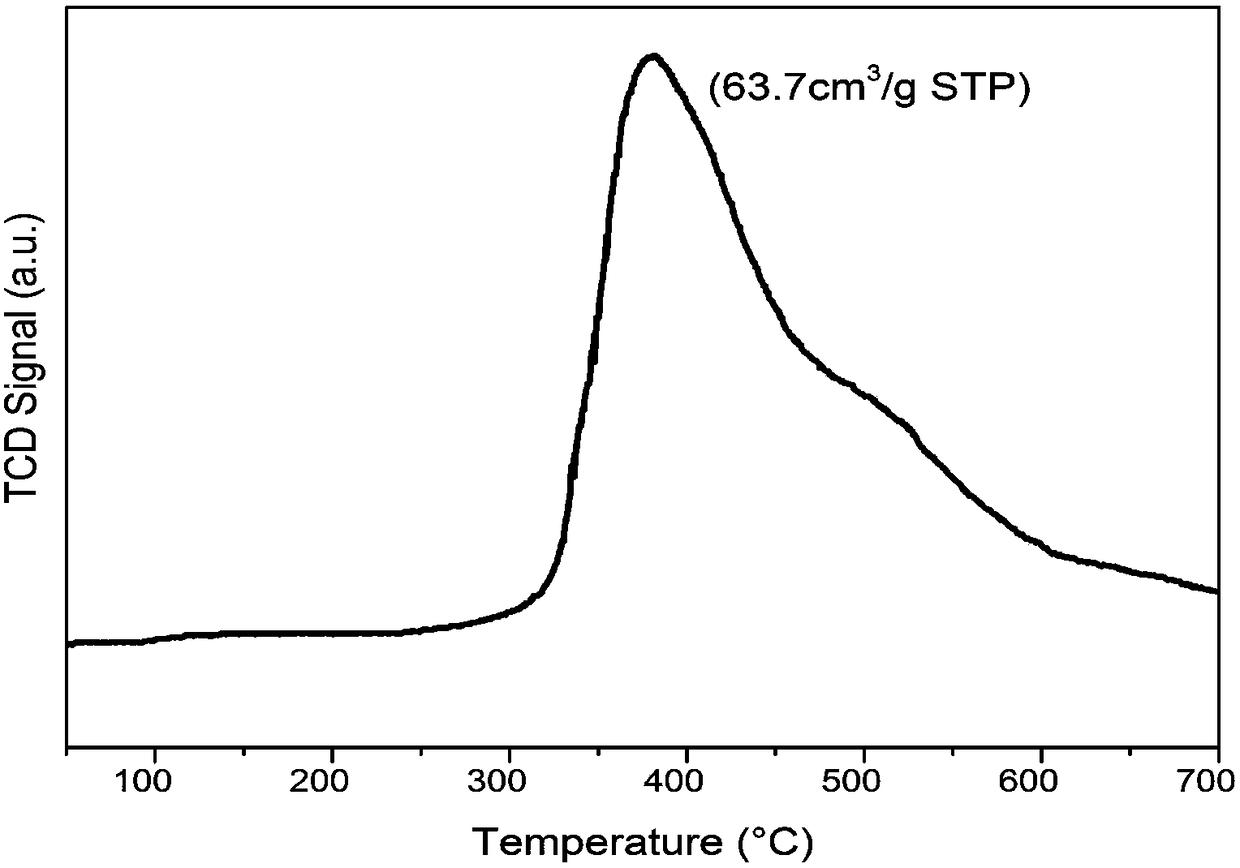
Porous zirconium pyrophosphate catalyst and application thereof to piperaldehyde hydrogenation reaction
A zirconium pyrophosphate and catalyst technology, applied in physical/chemical process catalysts, organic chemistry, chemical recovery and other directions, can solve the problems of easy separation and reuse of catalysts that do not meet the requirements, cannot be recycled, and Ni is expensive, and achieves good catalysis. effect, easy recycling, highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of zirconium pyrophosphate: Na 4 P 2 o 7 10H 2 O and ZrOCl 2 ·8H 2 Each of O was dissolved in 25mL deionized water to form solutions with a concentration of 0.04mol / L and 0.08mol / L respectively (the ratio of zirconium to phosphorus was 1:1), and the obtained zirconium oxychloride solution was added dropwise to sodium pyrophosphate After 14-18 minutes, the solution was dropped, and the mixture was continuously stirred at room temperature for 10 hours. The white precipitate was separated by centrifugation at 8500r / min, washed with deionized water and ethanol three times to remove Na + and Cl - , dried at 80°C for 12h.
Embodiment 2
[0031] (1) Take by weighing 1mmol piperonal, 200mg of zirconium pyrophosphate catalyst (the ratio of zirconium to phosphorus is 1:1) joins in the reactor that fills 5mL isopropanol;
[0032] (2) The above-mentioned reactor was placed in an oil bath at 160° C. and stirred for 10 h. After the reaction was cooled, a small amount of the reacted solution was taken to measure the yield of piperonyl alcohol and the conversion rate of piperonal with GC.
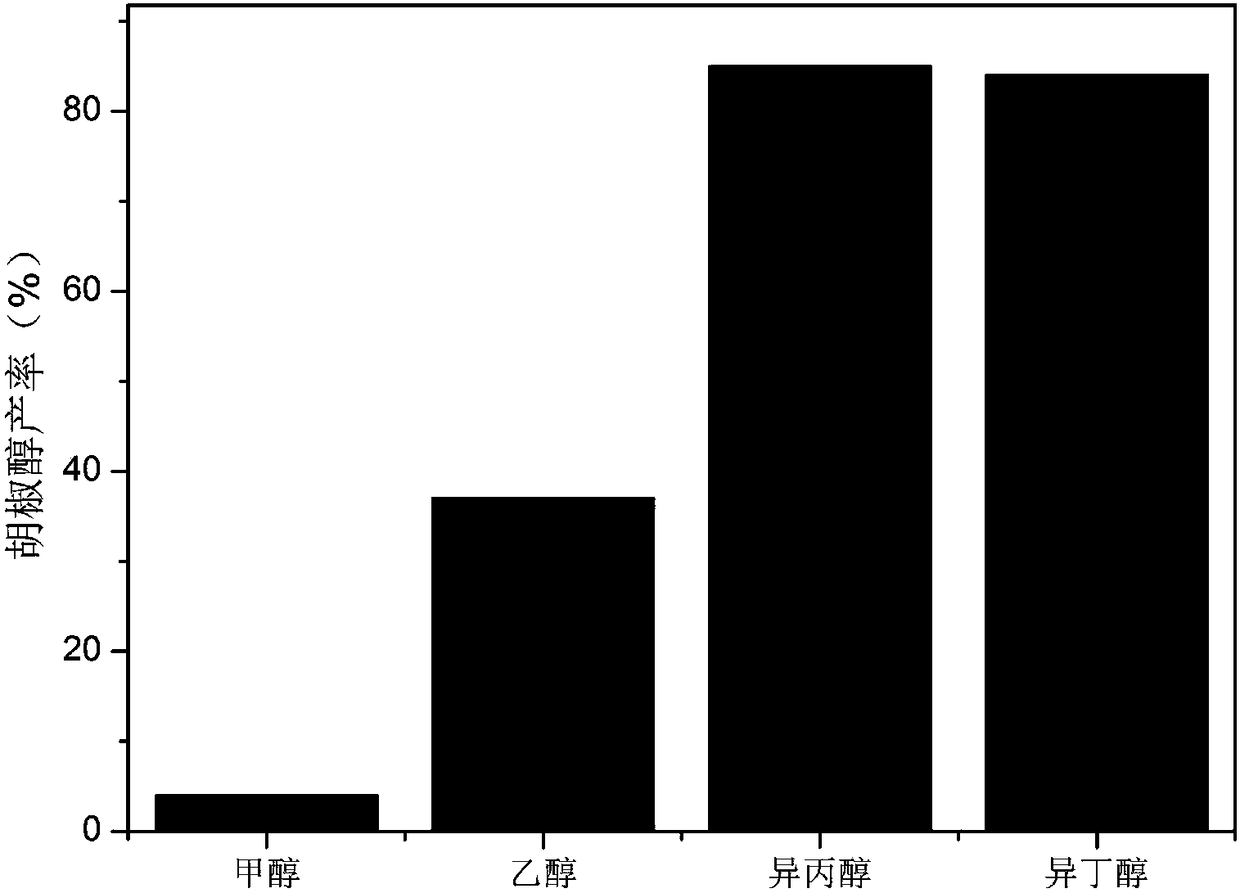
[0033] Change the isopropanol in (1) to other solvents, such as methanol, ethanol, isobutanol, and the reaction conditions of the remaining steps are the same. After getting a small amount of reaction, solution measures the conversion rate of piperonyl alcohol productive rate and piperonal with GC, the result is as follows image 3 Shown, corresponding methanol, ethanol, isopropanol and isobutanol make the conversion rate of the piperonal that solvent obtains be respectively 94%, 94%, 96%, 96%, and the productive rate of piperonyl al...
Embodiment 3
[0035] (1) Take by weighing 1mmol piperonal, 200mg of zirconium pyrophosphate catalyst (the ratio of zirconium to phosphorus is 1:1) joins in the reactor that fills 5mL isopropanol;
[0036] (2) The above-mentioned reactor was placed in an oil bath at 160° C. and stirred for 10 h. After the reaction was cooled, a small amount of the reacted solution was taken to measure the yield of piperonyl alcohol and the conversion rate of piperonal with GC.
[0037] Change the amount of zirconium pyrophosphate in (1) from 200 mg to 50, 100, 150, and 250 mg, and the reaction conditions of the remaining steps are the same. Cooling after completion of the reaction, get a small amount of reaction back solution and measure the conversion rate of piperonyl alcohol productive rate and piperonal with GC, the result is as follows Figure 4Shown, the addition of zirconium pyrophosphate amount is 50,100,150,200 and the conversion rate of the piperonal that 250mg obtains is respectively 46%, 58%, 81%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com