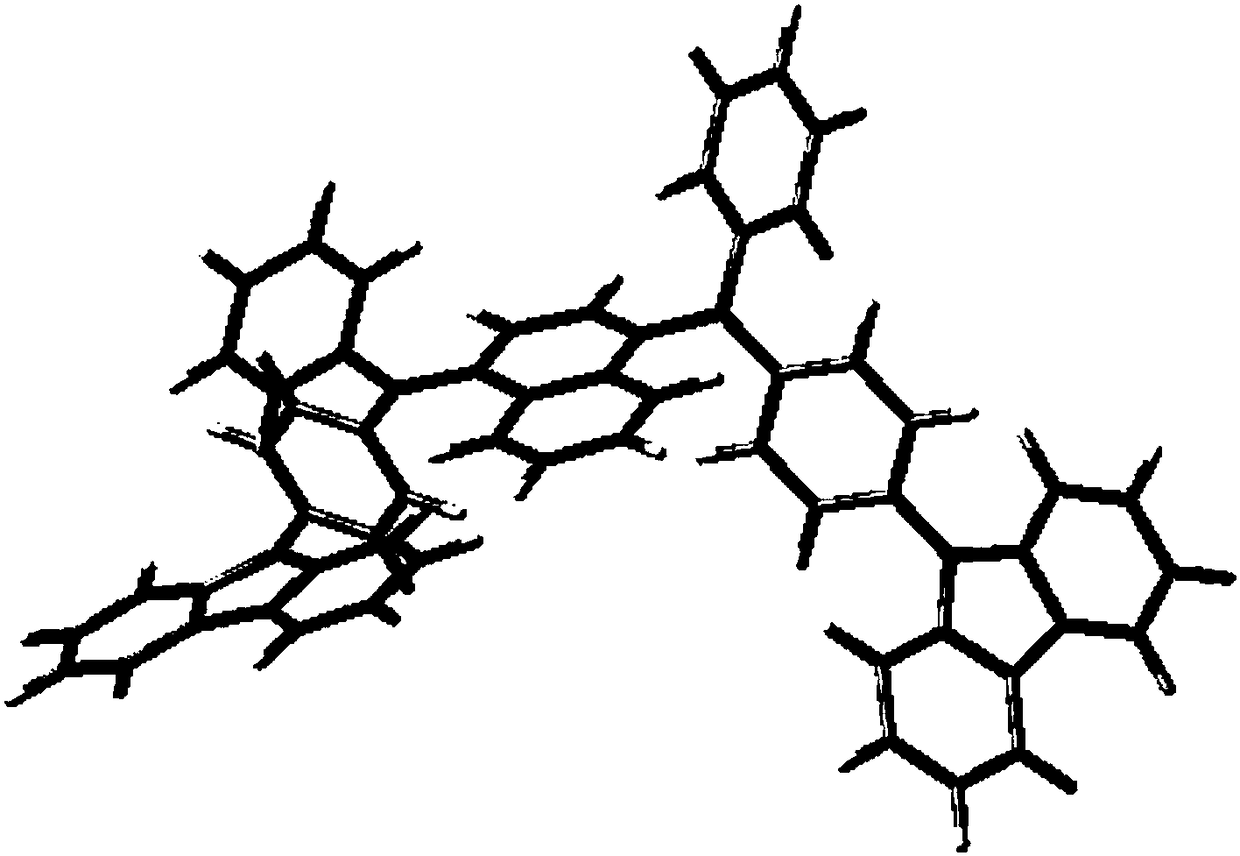
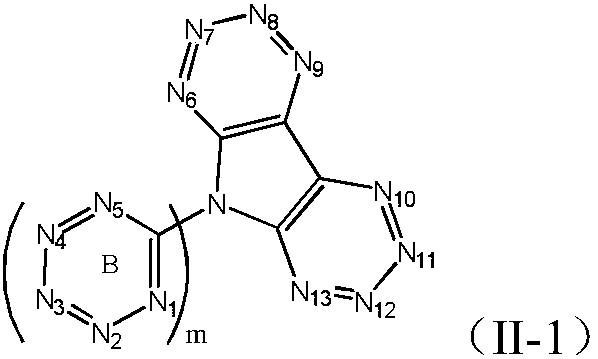
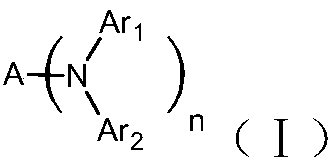Symmetrically-substituted aromatic amine compound and application in OLED device
An aromatic amine and compound technology, applied in the field of organic electroluminescent devices, can solve the problems of difficulty in commercialization, unclear mechanism, complex synthesis process, etc. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0088] The preparation methods of the compounds disclosed in the present invention are provided below. However, the present disclosure is not intended to be limited to any of the methods described herein. One skilled in the art can readily modify the described methods or utilize a different method to prepare one or more of the disclosed compounds. The following aspects are exemplary only, and are not intended to limit the scope of the present disclosure. Temperature, catalyst, concentration, reactant composition, and other process conditions may vary, and one skilled in the art of this disclosure can readily select appropriate reactants and conditions for a desired complex.
[0089] CDCl on a JEOL NMR instrument 3 or DMS0-d 6 Record at 400MHz in solution 1 H spectrum, recorded at 100MHz 13 C NMR spectrum, chemical shifts are referenced to residual protiated solvent. If CDCl 3 As a solvent, use tetramethylsilane (δ=0.00ppm) as the internal standard record 1 H NMR spectr...
preparation example
[0124] The manufacture of the device involved in the present invention adopts the conventional preparation method in the industry, and can be carried out in the following manner: first, select a suitable anode for introducing holes, and other materials can be evaporated on the surface of the anode to change the work function of the anode, and then organic layer, and then continue to evaporate the cathode to play the role of introducing electrons.
[0125] The organic layer can be one layer or multiple layers. Further, the organic layer includes a hole transport layer, a light emitting layer and an electron transport layer. The hole transport layer can also have a hole injection function, an electron blocking function or an exciton blocking function, and the electron transport layer can also have an electron injection function, a hole blocking function or an exciton blocking function, and the light-emitting layer can is a doped structure. When the light-emitting layer is a do...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com