(R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form, and preparation method and use thereof
A pyrrolidineacetamide, -4- technology, applied in the direction of organic chemical methods, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problem of less research on preparation methods and crystal forms, and no crystal form of pyrrolidineacetamide , disclosure and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Add 50 mg of (R)-4-hydroxy-2-oxo-1-pyrrolidine acetamide to 2 mL of sec-butanol, stir continuously, heat to 40°C, and filter to obtain a supersaturated solution, which is sealed and placed at -19 Cool and crystallize at ℃ for 24 hours to obtain colorless sand-like crystals.
Embodiment 2
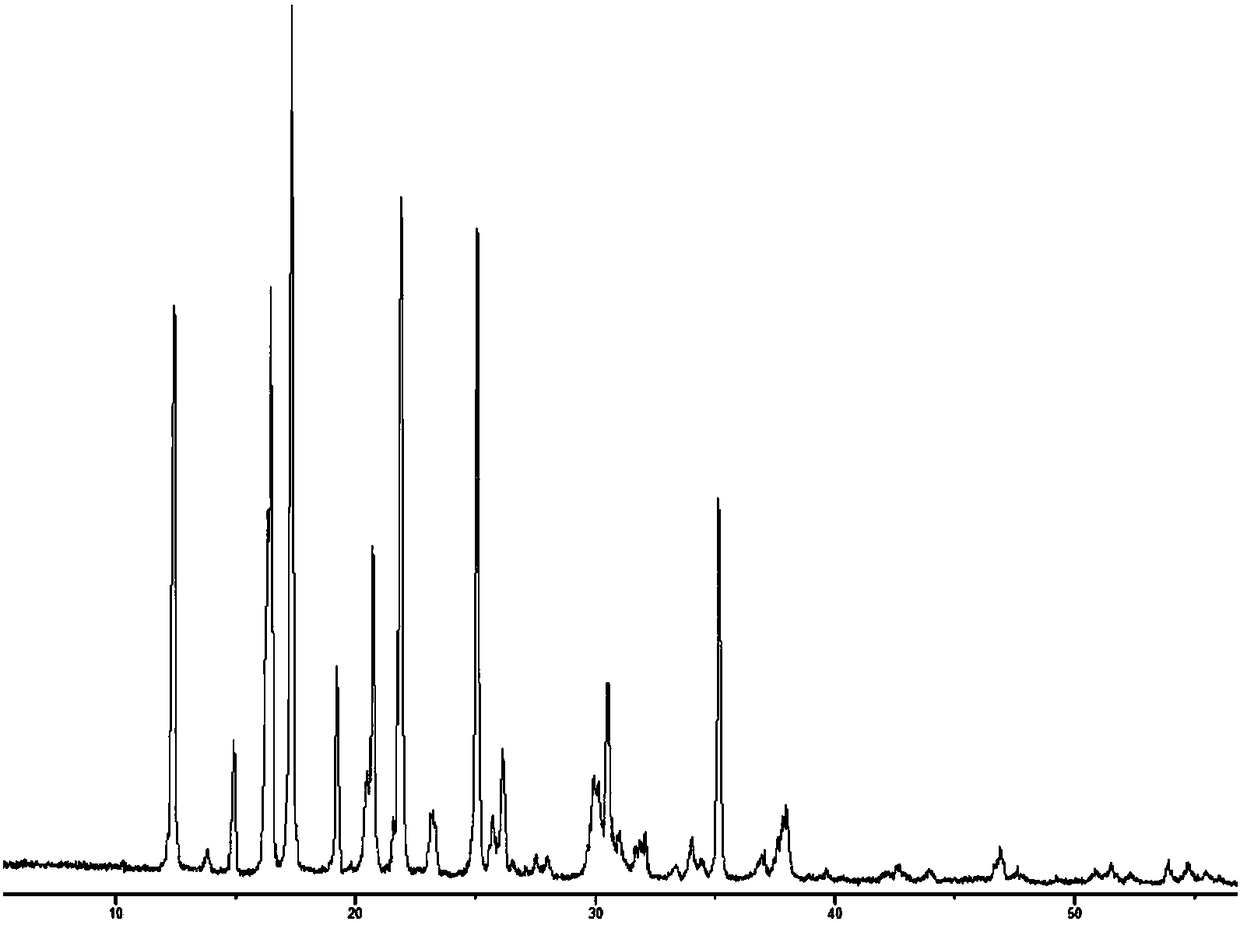
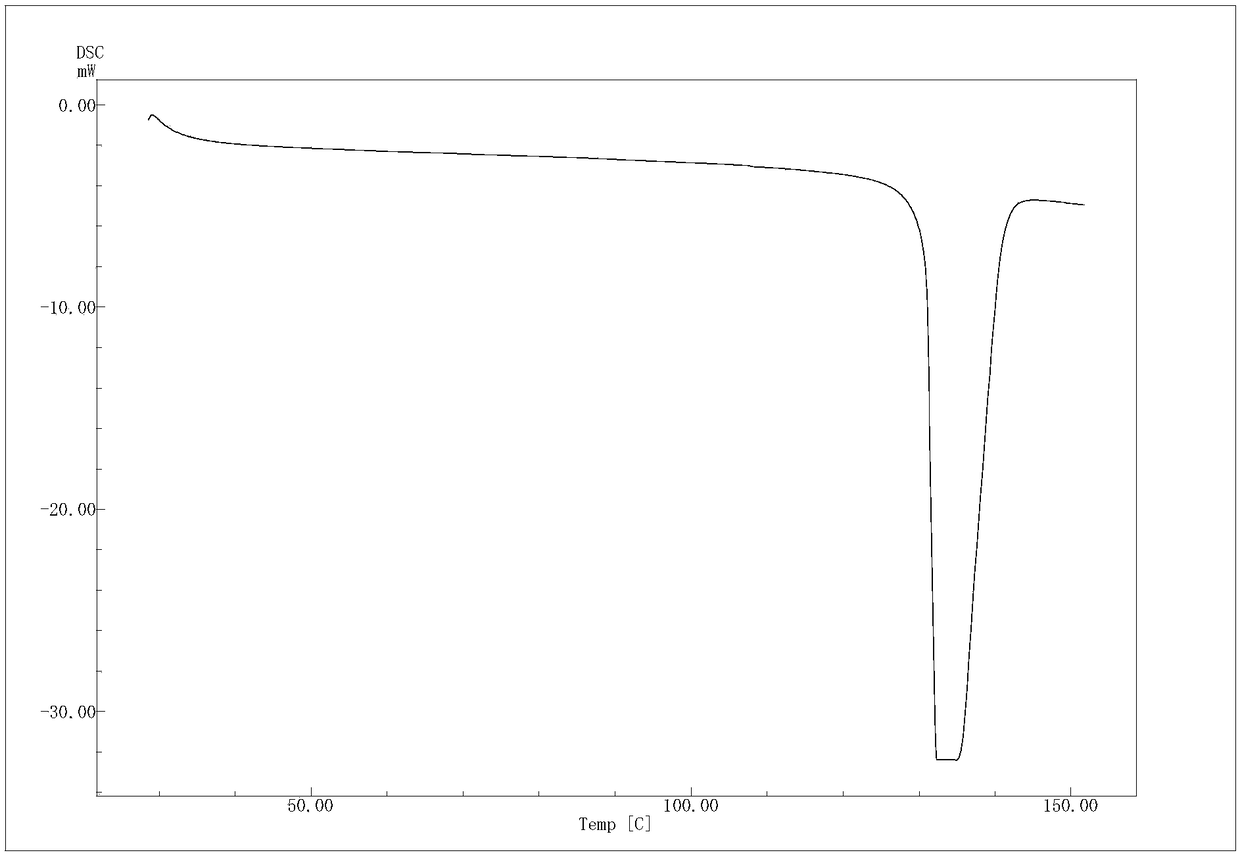
[0056]The (R)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide colorless sand granular crystal obtained in Example 1 is subjected to crystal parameter measurement, including powder diffraction measurement, infrared spectrum measurement, differential calorimetry measurement, Thermogravimetric assay. Test instrument conditions: The test uses a DX2500 X-ray diffractometer (Danfang Yuan Instrument, Liaoning) to analyze the pre-oxidized body and carbonized particles in each stage of the thermal stabilization process. Ni filter, CuKα as radiation source, X-ray wavelength λ=0.1541nm, acceleration voltage and current intensity are 40kV and 50mA respectively. Set the scanning interval to 0.02°, the scanning speed to 3° / min, and the scanning range to 5° to 45°. The crystals prepared in Example 1 have diffraction angles 2θ of 12.423, 14.928, 16.285, 16.465, 17.344, 19.198, 20.459, 20.707, 21.548, 21.889, 23.203, 25.054, 26.117, 29.913, 30.49, 35.1369, 37° peak, powder diffraction results as fi...
Embodiment 3
[0072] In 2mL of isopropanol, add 20mg of (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, stir continuously, heat to 50°C, and filter to obtain a supersaturated solution, which is sealed and placed in- Cool and crystallize at 17°C for 3 hours to obtain colorless sand-like crystals. Identified by the method of Example 2, it is (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com