Thio-carbonyl amide collector and preparation method and application thereof
A technology of thiocarbonyl amide and carbonyl amide, applied in solid separation, flotation, etc., to achieve the effects of shortened reaction time, good selectivity, and wide pH value of pulp
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Take 11.1g of phosphorus pentasulfide with a purity of 99% and 5.3g of sodium carbonate with a purity of 99.8%, add it to 100mL of ethyl acetate solution with a purity of 99.8%, and stir thoroughly at room temperature for 30 minutes; add 12.1g of benzene with a purity of 99% For formamide, the reaction bottle was placed in a microwave reactor, the power was adjusted to 300W, the temperature was set to 60°C, and the reaction time was set to 8 minutes. After the reaction was completed, thiobenzamide with a purity of 85.8% was obtained through concentration, extraction, concentration and filtration. The yield of thiobenzamide (based on benzamide) was 93.5%.
Embodiment 2
[0060] Take 22.2g of phosphorus pentasulfide with a purity of 99% and 11g of sodium carbonate with a purity of 99.8% and add it to 200mL of butyl acetate solution with a purity of 99.8%, and stir thoroughly at room temperature for 30 minutes; base acetamide, the reaction bottle was placed in a microwave reactor, the power was adjusted to 300W, the temperature was set to 60°C, and the reaction time was set to 12 minutes. After the reaction was finished, concentrated, extracted, concentrated, and filtered to obtain N-methylthioacetamide with a purity of 80.5%. The yield of N-methylthioacetamide (based on N-methylacetamide) was 94.6%.
Embodiment 3
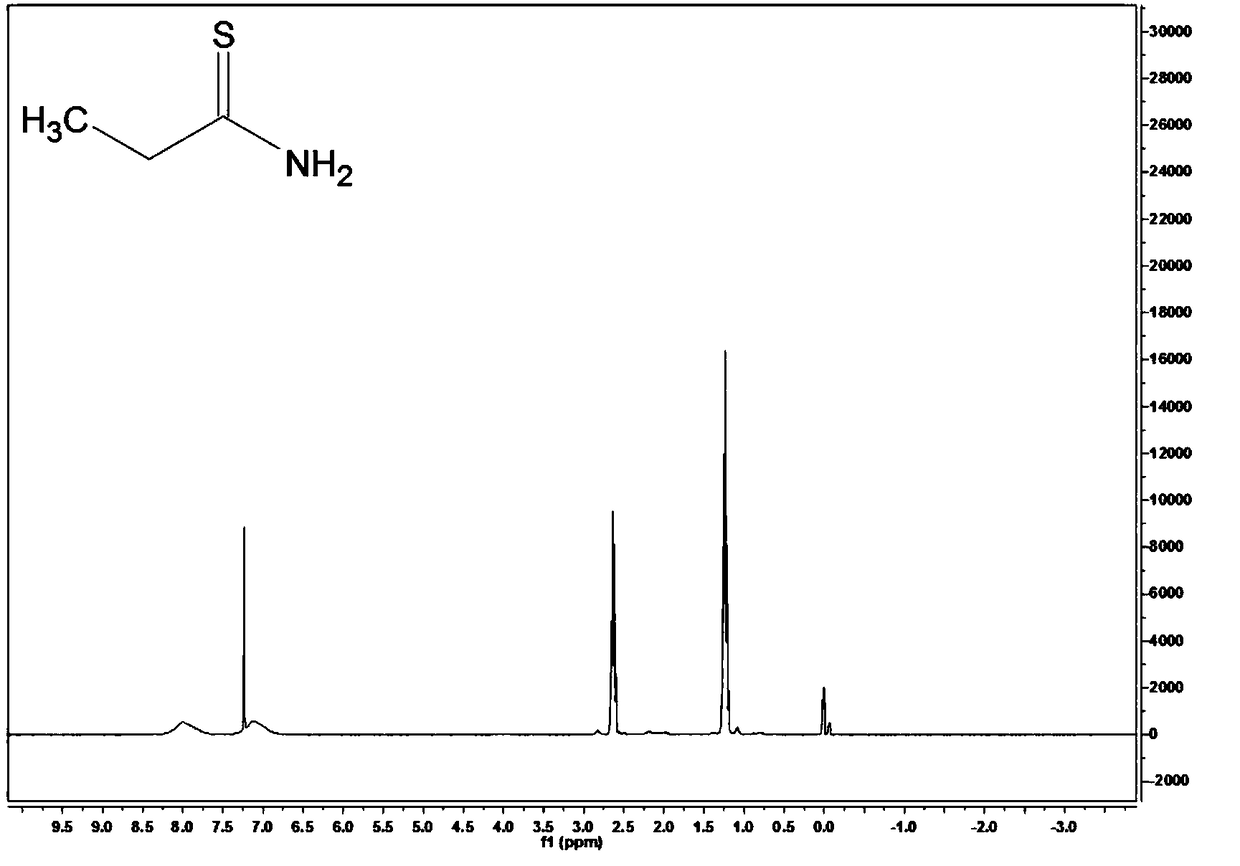
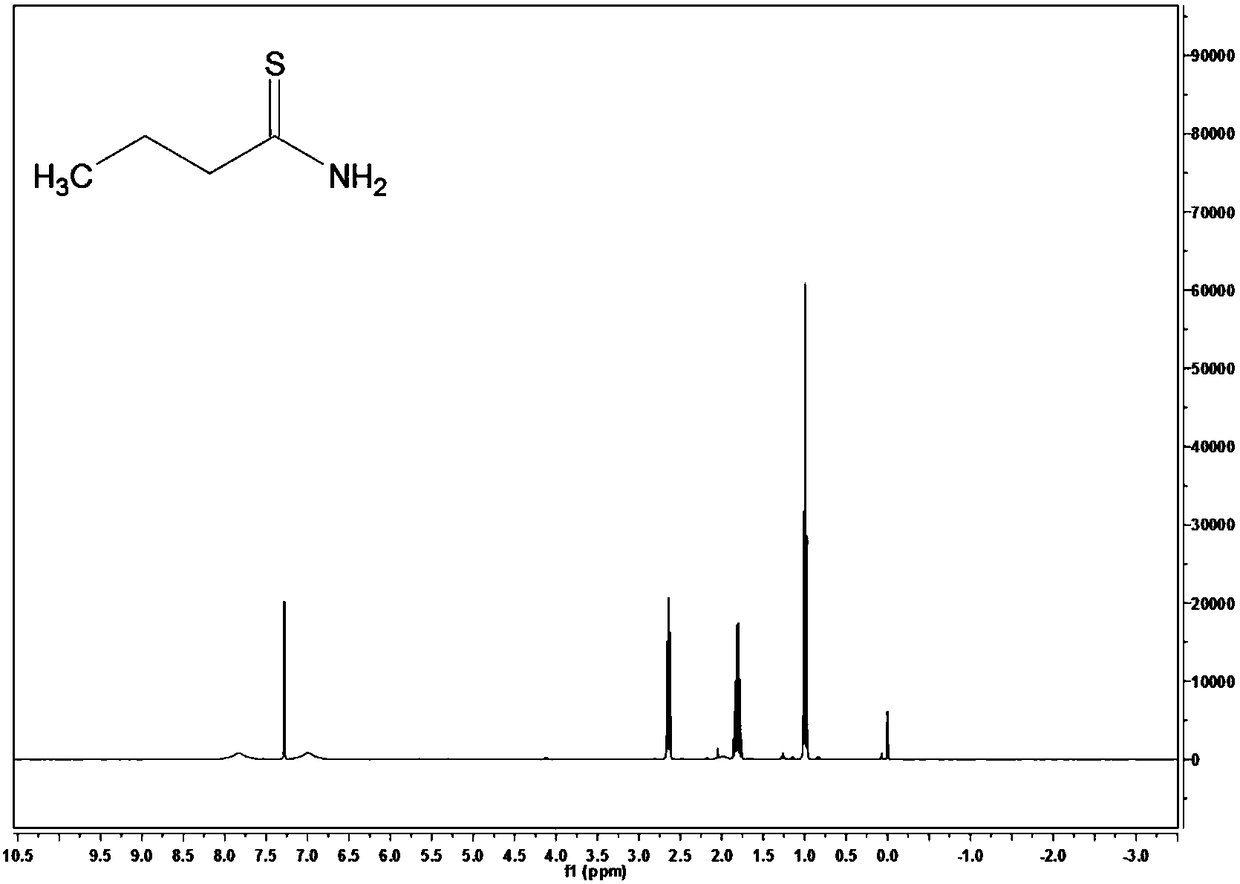
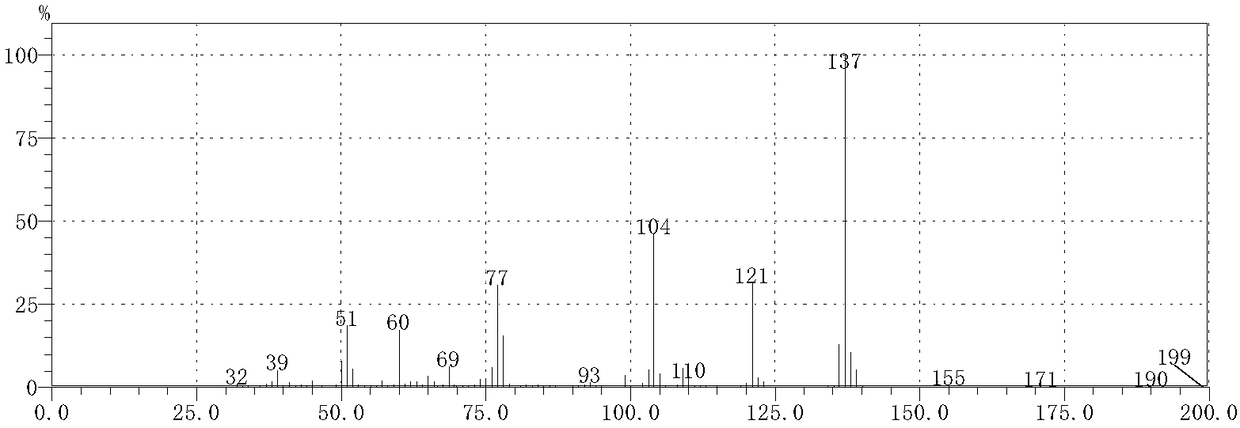
[0062] Take 11.1g of phosphorus pentasulfide with a purity of 99% and 6.9g of potassium carbonate with a purity of 99% and add it to 100mL of ethyl acetate solution with a purity of 99.8%, stir thoroughly at room temperature for 30 minutes; add 8.7g of butanamide with a purity of 98% , heated in a water bath for 7 hours, and the heating temperature was set at 50°C. After the reaction was completed, it was concentrated, extracted, concentrated and filtered to obtain thiobutyramide with a purity of 84.5%. The yield of thiobutyramide (based on butanamide) was 87.9%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com