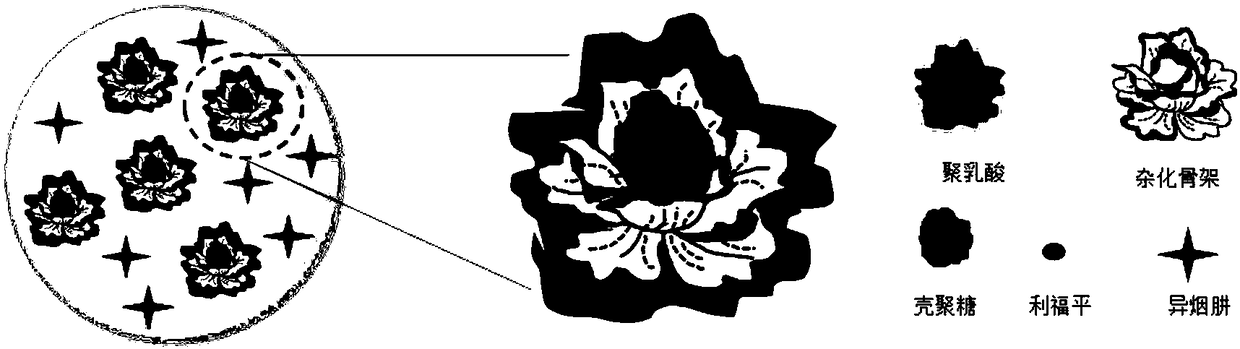
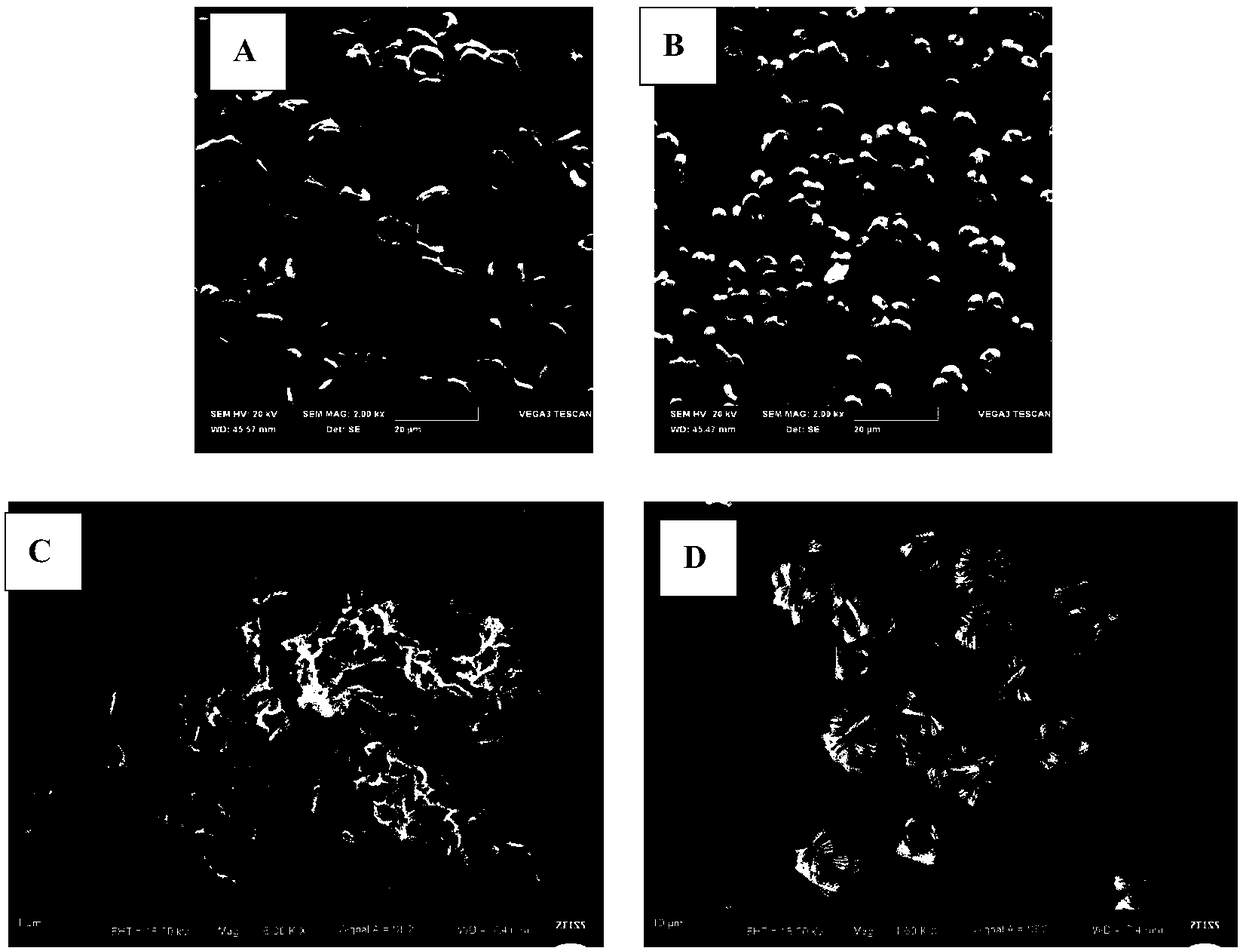
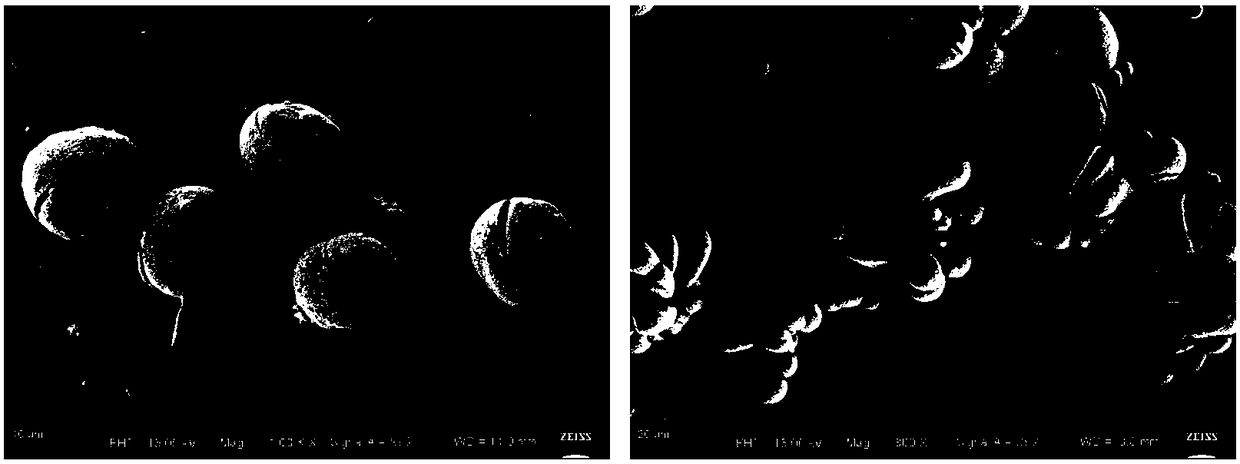
Bone tuberculosis medicine controlled-release microsphere with bone repairing function and preparation method
A technology for drug controlled release and bone tuberculosis, which is applied in the directions of medical preparations, pharmaceutical formulations, and drug combinations containing active ingredients, can solve the problem of multiple antibacterial drugs being loaded on the same carrier, etc., and achieves application potential and great value. Biocompatible, highly targeted effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Preparation of Novel Bone Tuberculosis Drug Controlled Release Microspheres with Bone Repair Function
[0043]Weigh 0.0381g of collagen and 0.0019g of bone morphogenic protein (BMP) and dissolve them in 200mL of phosphate buffered saline to obtain solution I; weigh 0.8g of zinc acetate and 0.2g of calcium acetate and dissolve them in 10mL of deionized water to obtain solution II; Add solution I to the Erlenmeyer flask, add solution II to it under agitation, react at room temperature for 3 hours, centrifuge, wash with water, freeze-dry to obtain the hybrid skeleton; weigh 0.2g polylactic acid, 0.01g rifampicin and 0.1g Dissolve and disperse the hybrid skeleton into 10mL of dichloromethane to obtain solution III; prepare 100mL of sodium dodecylsulfonate aqueous solution with a concentration of 1.5g / L, add it to a three-necked flask, add solution III to it under mechanical stirring, and store at room temperature Volatilize for 5 hours, centrifuge, wash with wate...
Embodiment 2
[0044] Example 2: Preparation of Novel Bone Tuberculosis Drug Controlled Release Microspheres with Bone Repair Function
[0045] Weigh 0.0952g human serum albumin and 0.0048g bone growth peptide (OGP) and dissolve in 200mL phosphate buffered saline solution to obtain solution I; weigh 1.12g zinc nitrate and 0.28g calcium chloride and dissolve in 10mL deionized water to obtain Solution II; add solution I to the Erlenmeyer flask, add solution II to it under stirring condition, react at room temperature for 2 hours, then centrifuge, wash with water, and freeze-dry to obtain the hybrid skeleton; weigh 0.1g polylactic acid, 0.015g rifampic acid Dissolve and disperse 0.05g of the hybrid skeleton in 5mL of dichloromethane to obtain solution III; prepare 100mL of sodium dodecylsulfonate aqueous solution with a concentration of 1.5g / L, add it to a three-necked flask, and add the solution to it under mechanical stirring III, volatilized at room temperature for 6 hours, centrifuged, wash...
Embodiment 3
[0046] Example 3: Preparation of Novel Bone Tuberculosis Drug Controlled Release Microspheres with Bone Repair Function
[0047] Weigh 0.0571g lysine and 0.0029g transforming growth factor family (TGFs) and dissolve in 200mL phosphate buffer solution to obtain solution I. ; Weigh 1.5g of zinc chloride and 0.3g of calcium gluconate and dissolve them in 15mL of deionized water to obtain solution II; add solution I to the Erlenmeyer flask, add solution II to it under stirring conditions, react at room temperature for 1 hour, and centrifuge , washed with water, and freeze-dried to obtain the hybrid skeleton; weigh 0.21g of polylactic acid, 0.014g of rifampicin and 0.105g of the hybrid skeleton to dissolve and disperse them in 7mL of dichloromethane to obtain solution III; configure the concentration of 1.5g / L Add 100 mL of sodium dodecylsulfonate aqueous solution into a three-necked flask, add solution III to it under mechanical stirring, volatilize at room temperature for 8 hours...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com