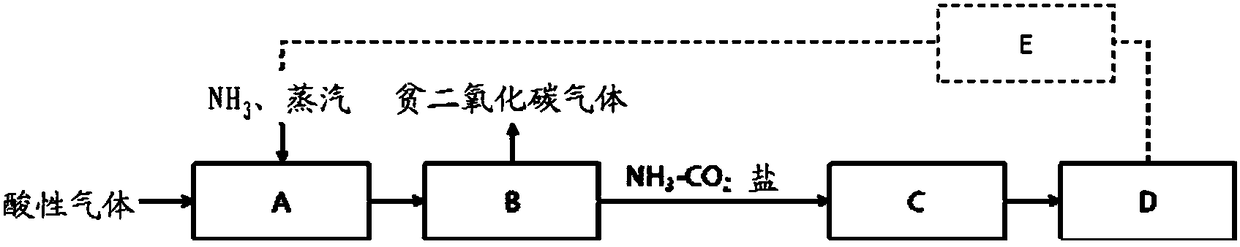
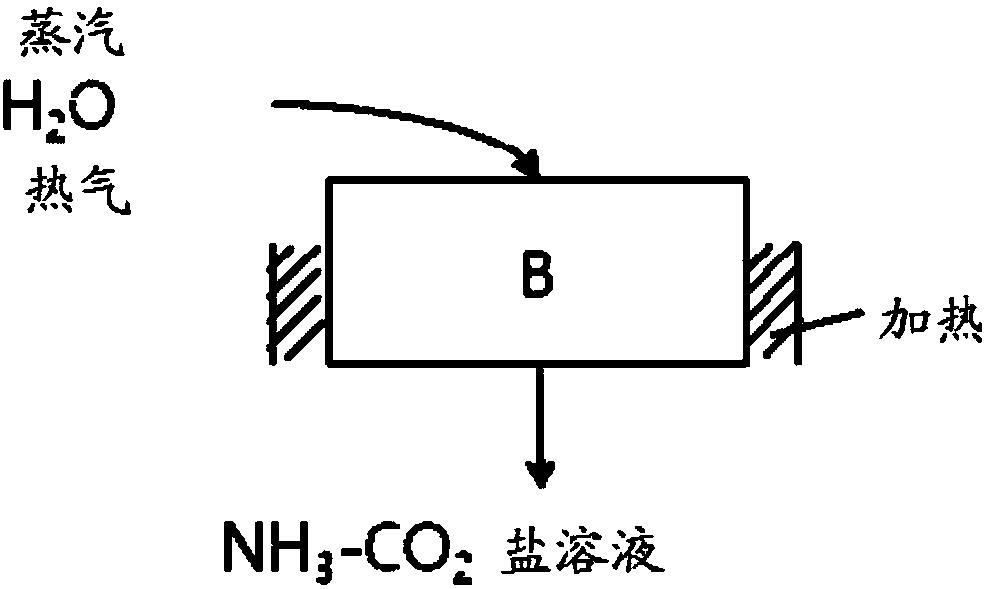
Method for removing carbon dioxide in acidic gas and apparatus therefor
A carbon dioxide and acid gas technology, applied in the removal of gas pollutants, dry distillation gas discharge devices, chemical instruments and methods, etc., can solve the problems of difficult circulation of reactants, etc., to improve economy, improve process efficiency, The effect of reducing the load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Then, in Example 1 of the present application, ammonia was added to the acid gas, and the molar ratio of carbon dioxide to ammonia in the entire mixed gas flow was adjusted to 0.7.
[0068] The gas stream to which the ammonia was added was indirectly cooled to 30°C to generate the salt. At this point, the salt generated by the reaction is white solid ammonium bicarbonate (NH 4 HCO 3 )Salt.
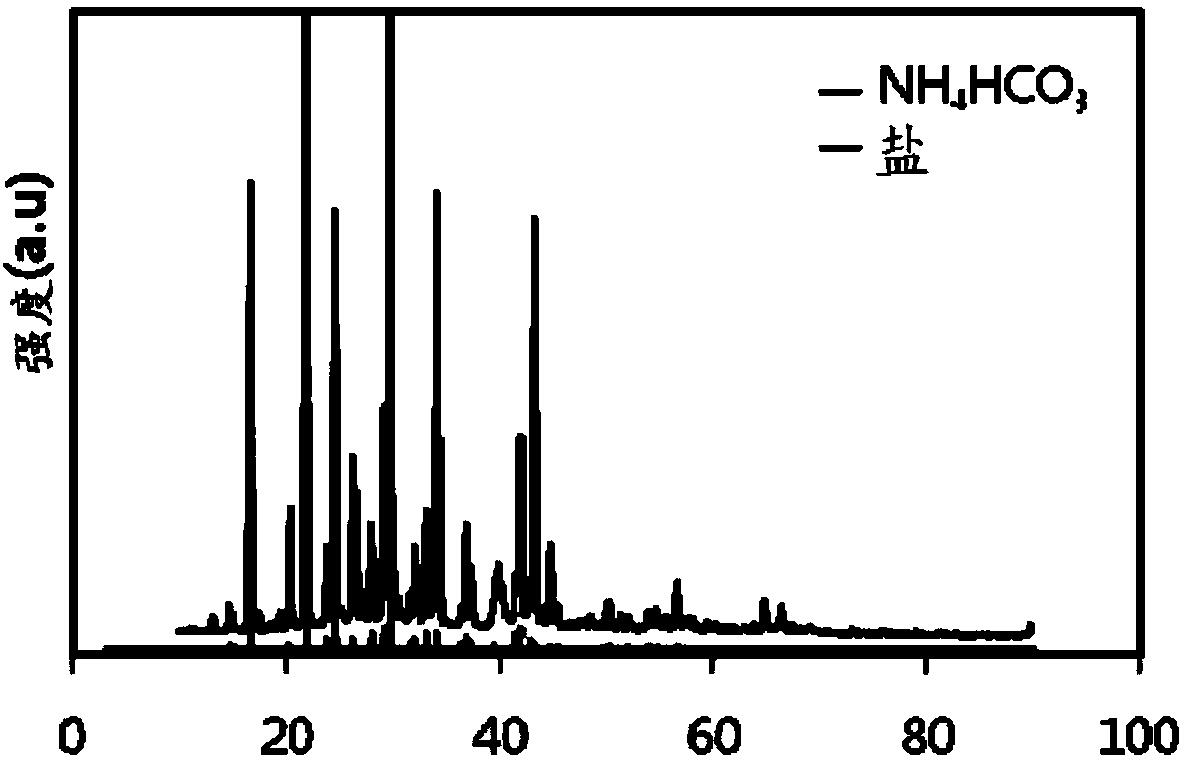
[0069] More specifically, image 3 It is a view analyzing the ammonium bicarbonate salt of Example 1 by X-ray diffraction (XRD). Such as image 3 Shown, with the result of X-ray diffraction analysis, confirm that the white solid salt of embodiment 1 is ammonium bicarbonate. More specifically, the image 3 Among the peaks shown in , the low-intensity peak represents ammonium bicarbonate (NH 4 HCO 3 )Salt.
[0070] The salt is then drained and removed as a slurry using steam.
[0071] The discharged slurry is heated by adding high-temperature nitrogen and steam, and is decom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com