Application of trialkyl naphthalene sulfonic acid, synergistic extraction agent containing trialkyl naphthalene sulfonic acid, as well as preparation and application thereof
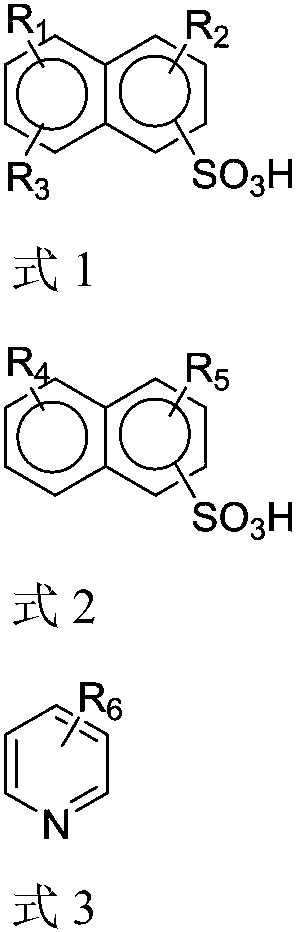
A technology of alkyl naphthalene sulfonic acid and dialkyl naphthalene sulfonic acid is applied in the field of extractant for metal extraction, which can solve the problems of slow extraction phase separation rate, etc. Effect of Clarification Rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0062] Synthetic example (long carbon chain trialkyl naphthalene sulfonic acid synthetic embodiment):
[0063] Taking the synthesis of alkyl dinonyl naphthalene sulfonic acid as a typical long carbon chain trialkyl naphthalene sulfonic acid as an example, under the protection of dry high-purity nitrogen, 5g (0.037mol) aluminum trichloride solid catalyst was added to 100g nitric acid Benzene solvent, stirred at 20°C for 0.5h, fully dissolved and added 99g (0.26mol) of dinonylnaphthalene (the substitution position of the nonyl group is not required), slowly added 0.275mol long Carbon chain brominated alkanes or alkenes (raw materials described in Table 1), then continue to stir the reaction for 1h, add 14.6g cold water to quench after the end of the reaction, then leave to separate layers, and the upper organic phase is washed with an equal volume of 10% NaOH aqueous solution Three times, and then washed with deionized water until neutral, and then the washed organic phase was d...
Embodiment 1
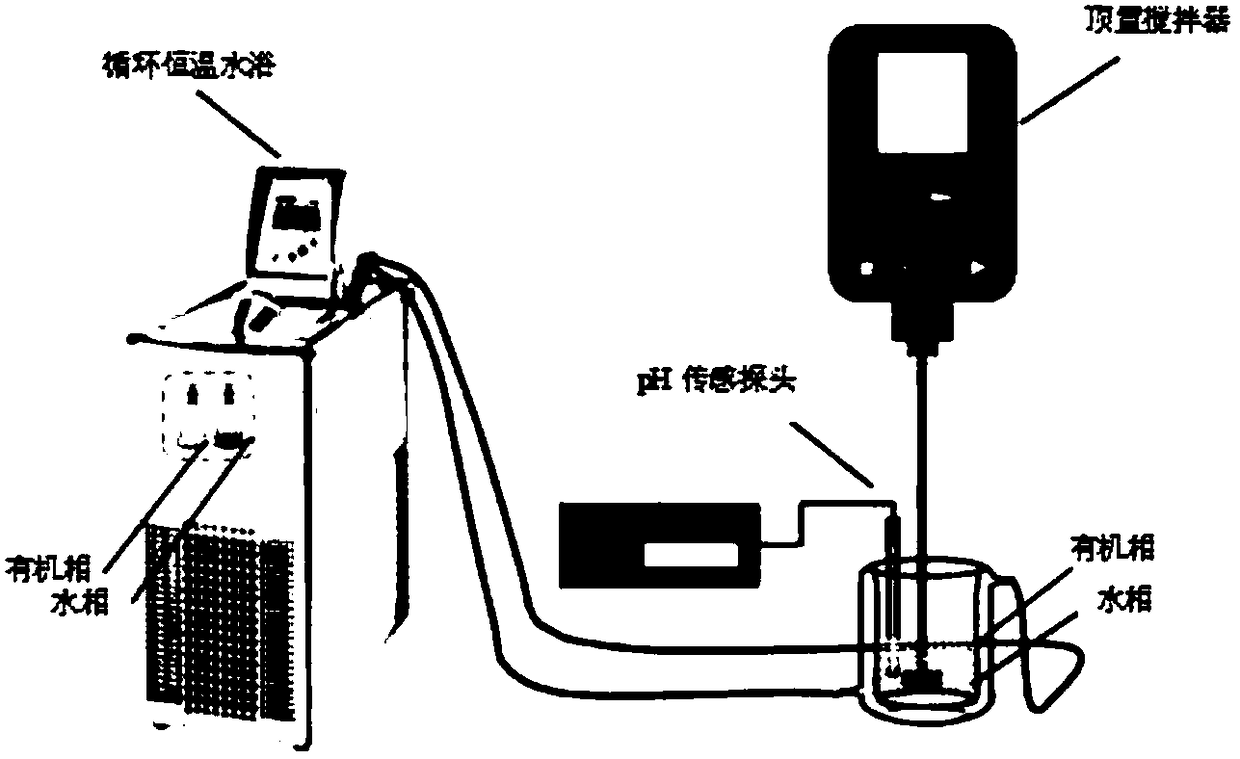
[0092] Taking trinonylnaphthalenesulfonic acid as a typical long carbon chain trialkylnaphthalenesulfonic acid as an example, add trinonylnaphthalenesulfonic acid to dinonylnaphthalenesulfonic acid / 4-pyridinecarboxylate isooctyl synergistic extractant, When its addition is 40% (mol percent) of the total amount of sulfonic acid groups in the organic phase, investigate the extraction effect of the simultaneous direct extraction of nickel and cobalt in the acidic polymetallic solution by the extraction temperature, the extraction clarification rate, the amount of solubilized water in the loaded organic phase and the effect of reverse micelle morphology.
[0093] Get the trinonylnaphthalenesulfonic acid synthesized in 10.2g synthetic examples (the product that 1-C case in table 1 obtains, wherein trinonylnaphthalenesulfonic acid 0.012mol, dinonylnaphthalenesulfonic acid 0.006mol), add 5.5g Dinonylnaphthalenesulfonic acid (0.012mol) and 14.1g isooctyl 4-pyridinecarboxylate (0.06mol...
Embodiment 2
[0102] Taking trinonylnaphthalenesulfonic acid as an example, add long carbon chain trialkylnaphthalenesulfonic acid to dinonylnaphthalenesulfonic acid / 4-pyridinecarboxylate isooctyl synergistic extractant, and its addition amount is the sulfonic acid in the organic phase When the total amount of groups is 40% (mole percent), the influence of concentration of extractant on simultaneous direct extraction of nickel and cobalt in acidic multi-metal solution, extraction clarification rate, loading organic phase solubilizing water and reverse micelle morphology was investigated.
[0103] Get the synthetic trinonylnaphthalenesulfonic acid (the product that the 1-C case in table 1 obtains in the 51g synthesis example, wherein trinonylnaphthalenesulfonic acid 0.06mol, dinonylnaphthalenesulfonic acid 0.03mol), add 27.5g dinonylnaphthalenesulfonic acid Nonyl naphthalene sulfonic acid (0.06mol) and 60.5g 4-pyridinecarboxylate (0.06mol), are configured into the mol ratio 1 of ester group i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com