A kind of preparation method of 3α-hydroxyl-5α, 14β-androst-15-en-17-one
A technology of hydroxy androstane and hydroxyl group, applied in the field of compound preparation, can solve the problems of low yield, insufficient utilization of raw materials, difficult separation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention will be further described below in conjunction with embodiment. The raw materials used in the present invention are all commercial raw materials, which can be purchased from the market.
[0020]
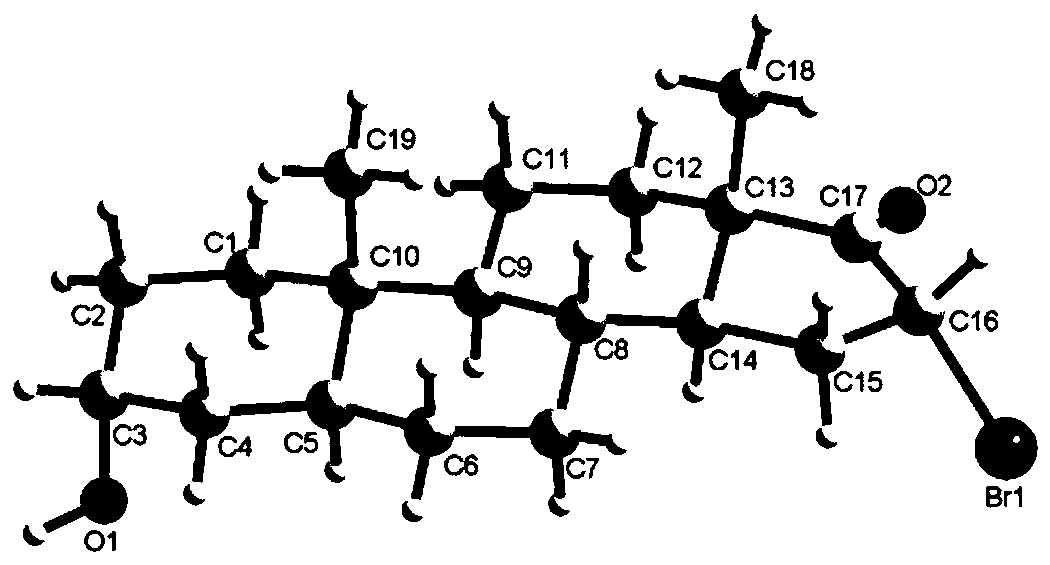
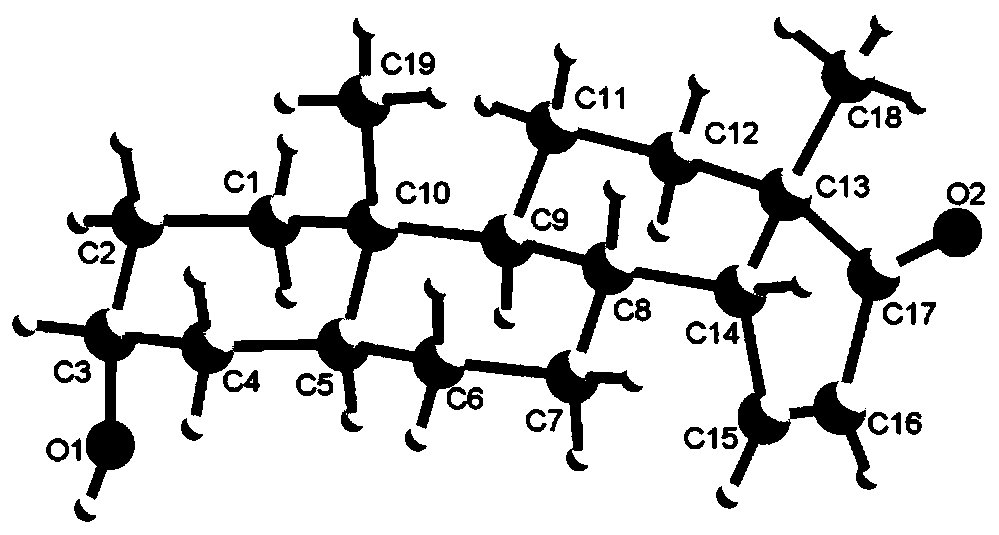
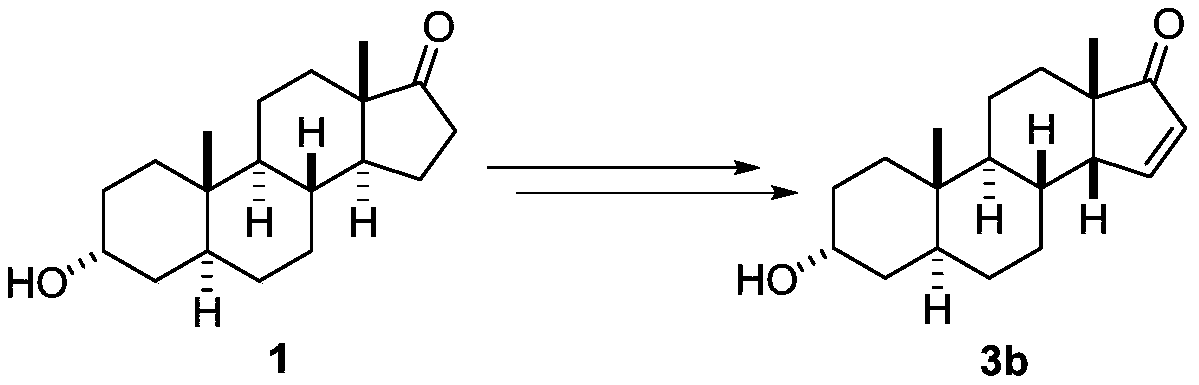
[0021] Scheme 1 Reaction equation for the synthesis of 3α-hydroxyandrost-14-en-17-one (3a) and 3α-hydroxy-14β-androst-15-en-17-one (3b)
[0022] Synthesis of 3α-Hydroxy-16α-Bromandrost-17-one (2)
[0023] Take 5.80g (20mmol) of 3α-hydroxy-5α-androst-17-one androsterone, 11.20g (50mmol) of copper bromide, and 100mL of methanol in a 250ml round-bottomed flask, heat up to reflux, and track the reaction by TLC. 8h raw materials completely reacted. Stop the reaction, evaporate the reaction solvent methanol under reduced pressure, then add 50 mL of water to the residue, extract and separate the liquids with dichloromethane (2×50 mL), wash the organic phase with 50 mL of water for 2-3 times, and wash with 25 mL of saturated sodium chloride solution for 1 -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com