Method for preparing (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form
A technology of pyrrolidine acetamide, -4-, applied in the directions of organic chemistry, organic chemistry, etc., can solve the problems of disclosure of preparation method, less research on preparation method and crystal form of pyrrolidine acetamide, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] In 150mL of methanol, add 10g of (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, stir continuously, heat to 50°C, dissolve completely, add 0.5g of activated carbon and stir for 30 minutes, filter to obtain methanol solution, the methanol solution was concentrated to a total volume of 20ml to obtain a saturated methanol solution, 30ml of ethanol was added, stirred and crystallized at 5°C, and filtered to obtain colorless sand-like crystals. Identified by the method of Example 2, it is (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form I.
Embodiment 2
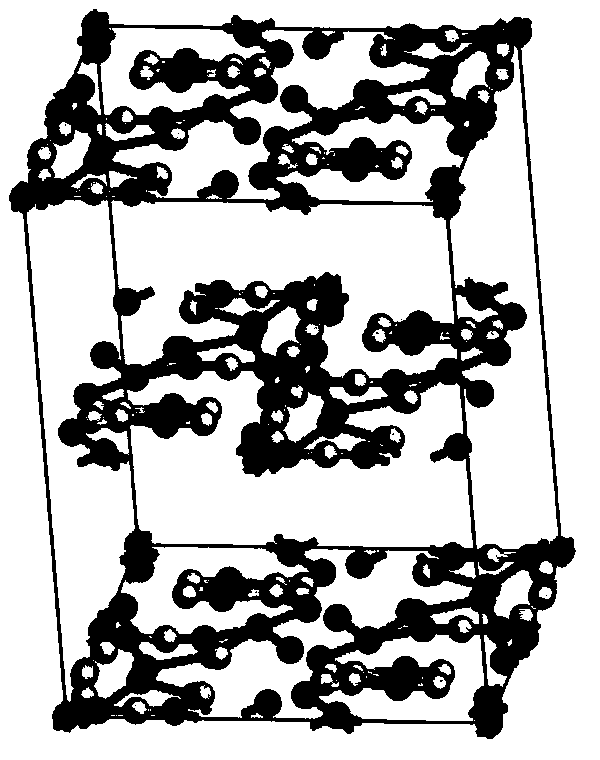
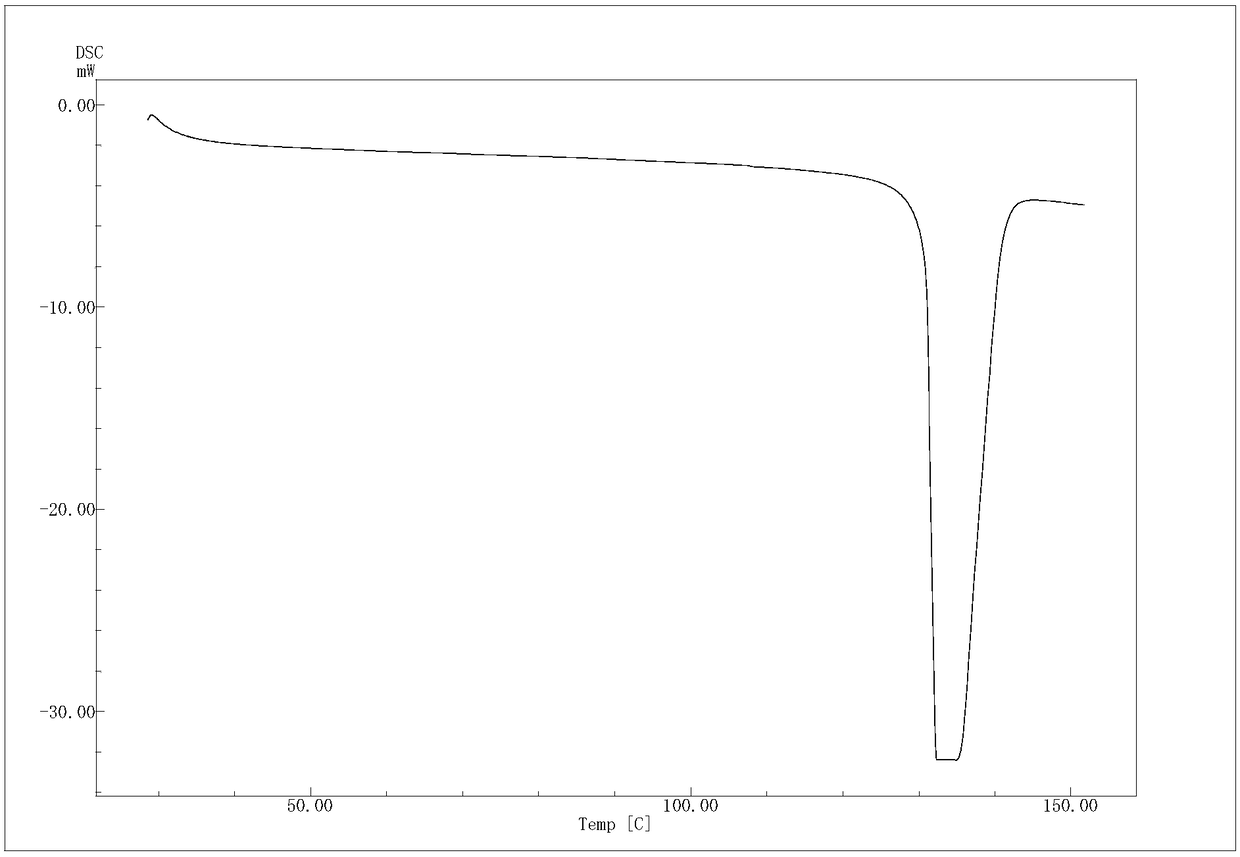
[0048] The (R)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide colorless sand granular crystal obtained in Example 1 is subjected to crystal parameter measurement, including powder diffraction measurement, infrared spectrum measurement, differential calorimetry measurement, Thermogravimetric assay. Test instrument conditions: The test uses a DX2500 X-ray diffractometer (Danfang Yuan Instrument, Liaoning) to analyze the pre-oxidized body and carbonized particles in each stage of the thermal stabilization process. Ni filter, CuKα as radiation source, X-ray wavelength λ=0.1541nm, acceleration voltage and current intensity are 40kV and 50mA respectively. Set the scanning interval to 0.02°, the scanning speed to 3° / min, and the scanning range to 5° to 45°. The crystals prepared in Example 1 have diffraction angles 2θ of 12.423, 14.928, 16.285, 16.465, 17.344, 19.198, 20.459, 20.707, 21.548, 21.889, 23.203, 25.054, 26.117, 29.913, 30.49, 35.1369, 37° peak, powder diffraction results as f...
Embodiment 3
[0064] In 150mL of methanol, add 8g of (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide, stir continuously, heat to 30°C, dissolve completely, add 0.4g of activated carbon and stir for 20 minutes, filter to obtain methanol solution, the methanol solution was concentrated to a total volume of 20ml to obtain a saturated methanol solution, 20ml of ethanol was added, stirred and crystallized at 4°C, and filtered to obtain colorless sand-like crystals. Identified by the method of Example 2, it is (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com