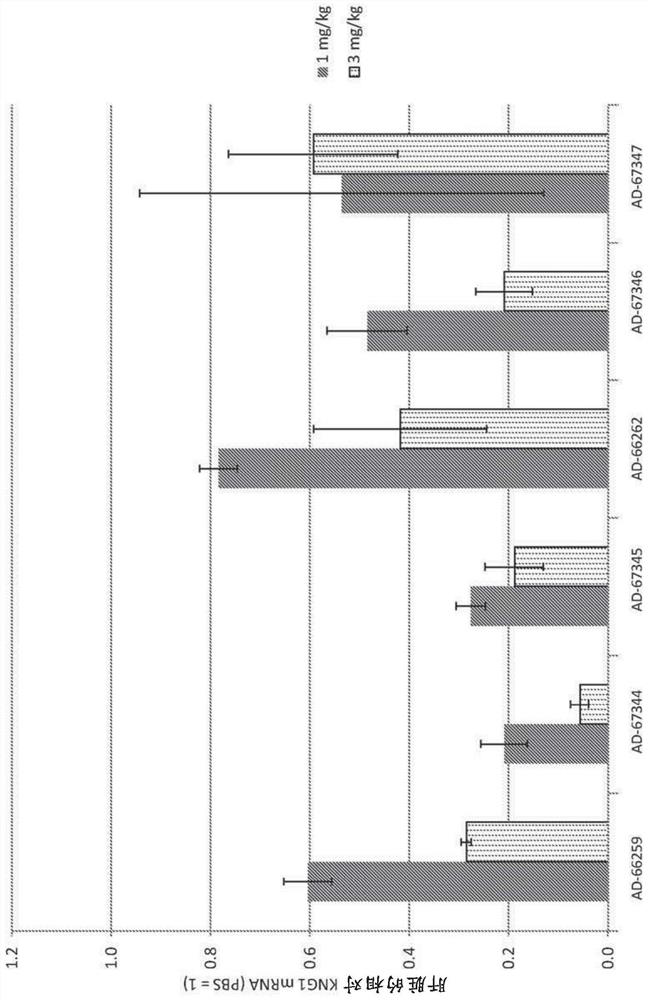
Factor xii (Hagemann factor) (f12), kallikrein b, plasma (Fletcher factor) 1 (klkb1) and kininogen 1 (kng1) irna compositions and methods of use thereof
A factor-inhibitor technology for the combination of factor XII (Hagemann factor) (F12), kallikrein B, plasma (Fletcher factor) 1 (KLKB1) and kininogen 1 (KNG1) iRNAs drug and its field of use, able to address negative effects, lack of safety, efficacy, and need for drug delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
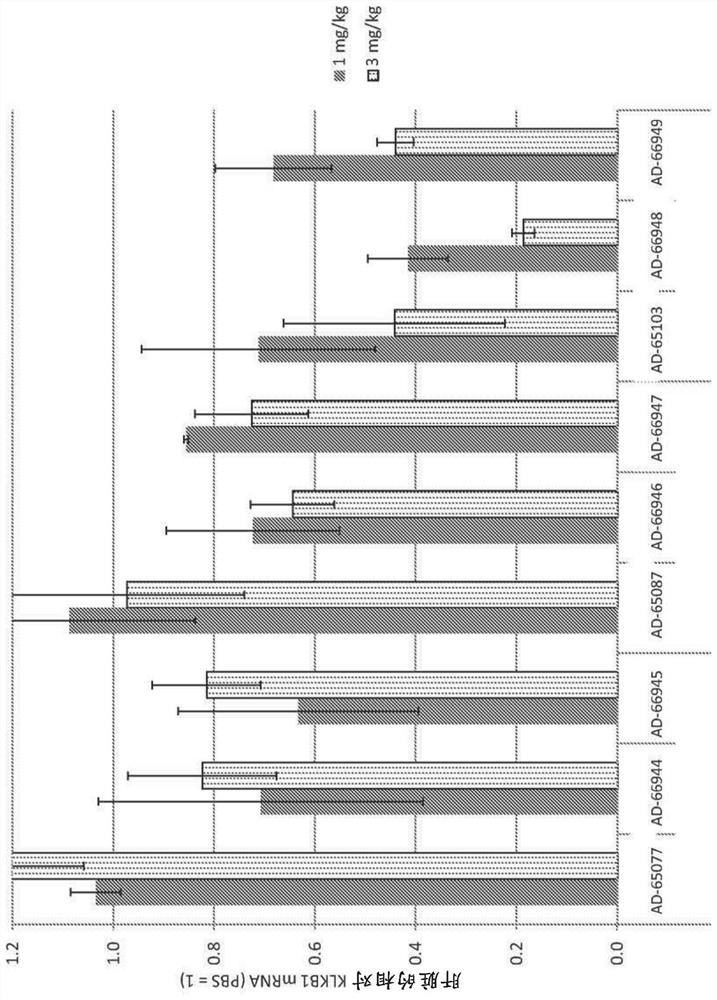
[0695] Example 1: Synthesis of KLKB1 iRNA
[0696] source of reagents
[0697] Herein, if the source of a reagent is not specified, the reagent can be obtained from any supplier of reagents for molecular biology in quality / purity for molecular biology applications.
[0698] transcript
[0699] siRNA design
[0700]For human KLKB1, "Kallikrein B, Plasma (Fletcher Factor) 1" (REFSeq Accession No. NM_000892.3, GI:78191797, GeneID:3818, SEQ ID NO:1 and SEQ ID NO.2) and KLKB1 xenologs from virulent species (Cynomolgus: RefSeq Accession No. XM_005556482, GI: 544436072; Rhesus: RefSeqJU329355, GI: 380802470; Mouse: RefSeqNM_008455, GI: 236465804; Rat RefSeqNM_012725, GI: 16213890) A targeted set of siRNAs was designed using custom R and Python scripts. Human KLKB1 RefSeq mRNA is 2252 bases in length. The rationale and methodology for this set of siRNA designs are as follows: Using a linear model that predicts direct measures of mRNA knockdown, based on over 20,000 distinct siRNA...
example 2
[0706] Example 2. In vitro screening of KLKB1 siRNA duplexes
[0707] Cell Culture and Transfection
[0708] Cos7 cells (ATCC, Manassas, VA) were grown at 37°C, 5% CO 2 They were grown to near confluence in DMEM (ATCC) supplemented with 10% FBS under atmosphere and released from the plate by trypsinization. Luciferase constructs were generated in psiCHECK2 plastids containing approximately 2.2 kb of human KLKB1 genomic sequence or 2.5 kb of xenologous mouse KLKB1 genomic sequence. Dual-luciferase plastids were each transfected with siRNA at 15x10 using Lipofectamine 2000 (Invitrogen, Carlsbad CA. cat#11668-019). 4 in the cell. For each well of a 96-well plate, 0.2 μl of Lipofectamine was added to 10 ng of plastid vector and single siRNA (Tables 3 and 4) in 14.8 μL of Opti-MEM, and the complex was left at room temperature for 15 minutes. The mixture was then added to the cells, which were resuspended in 80 [mu]l of fresh complete medium. After incubating the cells for 24 ...
example 3
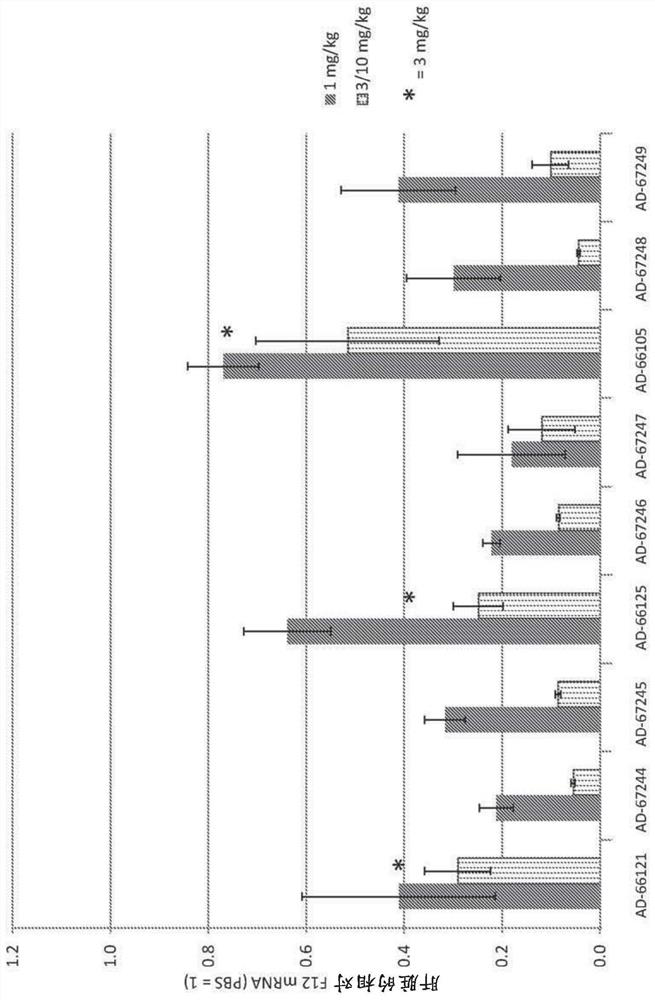
[0737] Example 3. Synthesis of F12iRNA
[0738] source of reagents
[0739] Herein, if the source of a reagent is not specified, the reagent can be obtained from any supplier of reagents for molecular biology in quality / purity for molecular biology applications.
[0740] transcript
[0741] siRNA design
[0742] For human F12, "Factor XII" (Human: NCBI refseqID NM_000505; NCBI GeneID: 2161) and F12 xenologs from virulent species (Cynomolgus: XM_005558647; Mouse: NM_021489; Rat: NM_001014006) were A targeted set of siRNAs was designed using custom R and Python scripts. Human F12RefSeq mRNA is 2060 bases in length. The rationale and methodology for this set of siRNA designs are as follows: Human F12 mRNA (containing the coding region and Predicted potency of each potential 19mer siRNA from position 50 to position 2060 (coding region and 3'UTR) of the 3'UTR. A subset of this F12 siRNA is designed to be a perfect or near-perfect match between humans, cynomolgus monkeys and r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com