Method for predicating toxicity MOA (mode of action) of pharmaceutical product
A technology of toxicity and pharmaceuticals, applied in chemical property prediction, special data processing applications, instruments, etc., to achieve the effect of lifting limitations, reducing development costs, and improving robustness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
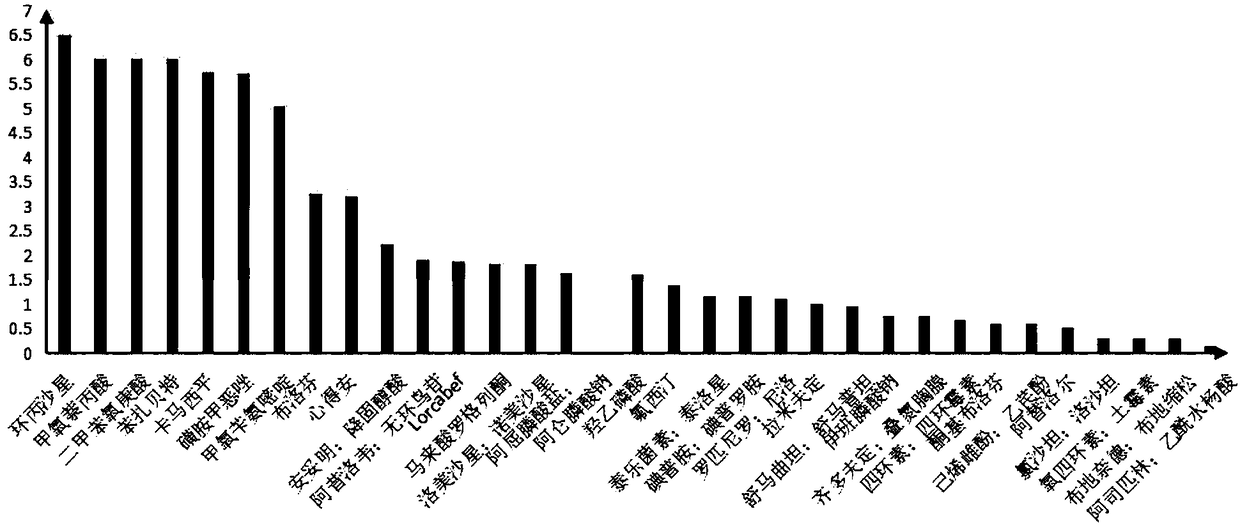
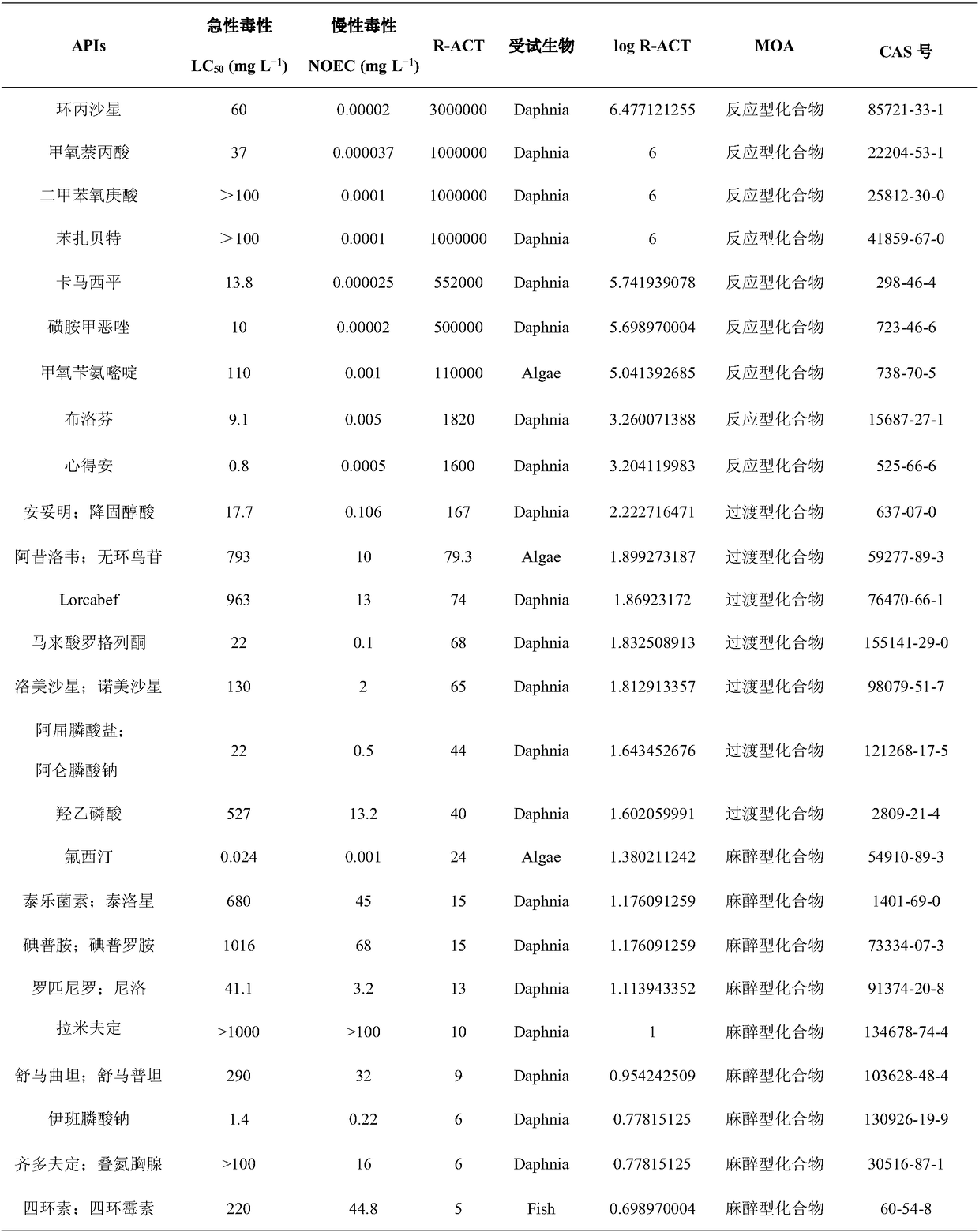
[0025] Taking 33 known medicines as an example, the corresponding acute and chronic data are obtained according to the acute toxicity test or the medicine information database, according to the formula Calculate the drug R-ACT and its logR-ACT, and classify the MOA of the drug according to the Log R-ACT value. When log R-ACT≤1.5, the MOA of the drug is an anesthetic compound. When 1.5figure 1 .
[0026] Table 1 Pharmaceutical R-ACT and its MOA
[0027]
[0028]
Embodiment 2
[0030] The method of Example 1 was used to evaluate the R-ACT and MOA of the same drug in different organisms. The evaluation results are shown in Table 2.
[0031] Table 2 R-ACT and MOA of the same drug in different organisms
[0032]
[0033]
[0034] Note: ▲-anesthetic compound ■-reactive compound ●-transitional compound
[0035] It can be seen from this embodiment that the same drug exhibits the same MOA for different organisms, and the screening method provided by the present invention has a high accuracy rate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com