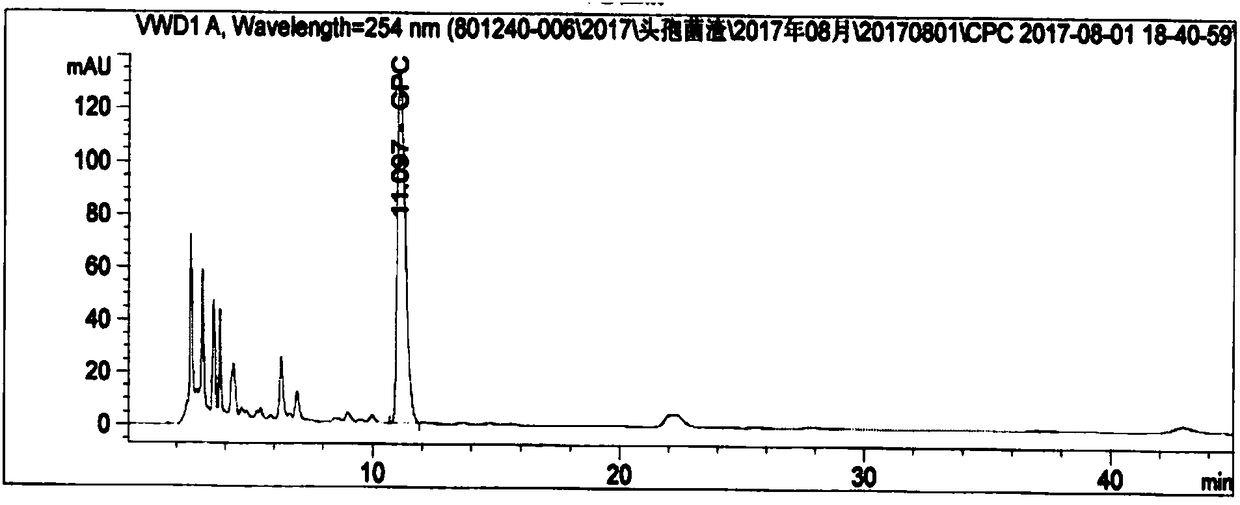
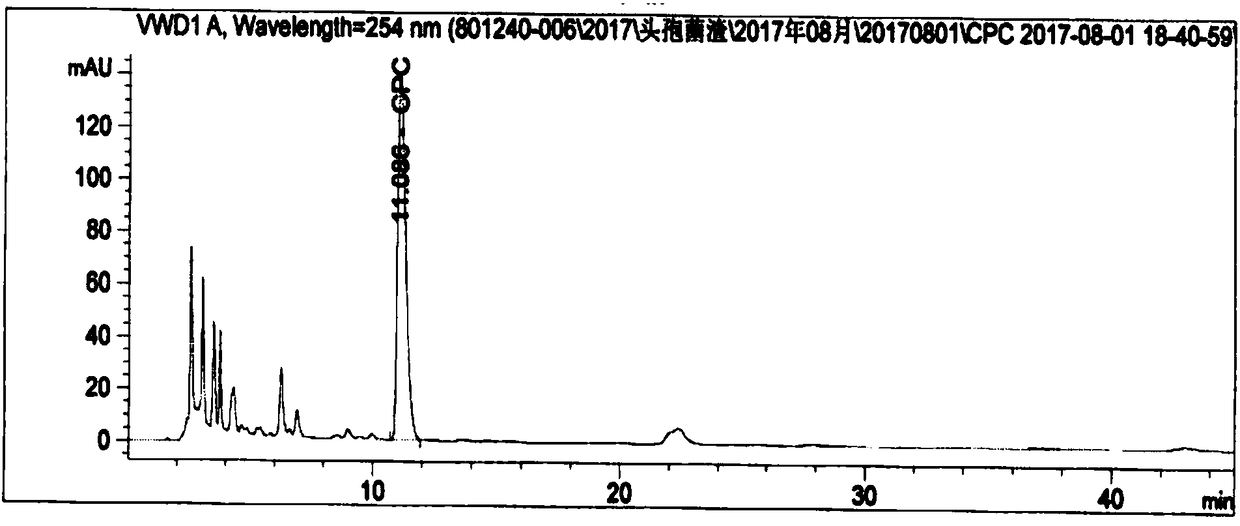
Method for detecting residual titer of cephalosporin C in cephalosporin residues
A method for measuring cephalosporins, which is applied in the field of determination of cephalosporin C residual potency in cephalosporin residues, can solve the problems of inaccurate detection of cephalosporin C content, inability to determine environmental hazards and impacts, and restrict bacteria residues Reuse of resources and other issues to achieve the effect of high accuracy, high sensitivity, and high separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Determination of residual potency of cephalosporin C in spray-dried inactivated cephalosporin residue
[0048] 1) Preparation of buffer
[0049] 0.1mol / L phosphate buffer (pH 5.0): Weigh 17.9g disodium hydrogen phosphate (Na 2 HPO 4 12H 2 O) (analytical pure) was dissolved in 500 ml of purified water, and the pH was adjusted to 5.0 with phosphoric acid (analytical pure).
[0050] 0.01mol / L sodium acetate buffer solution (pH 4.75): Weigh 0.4g sodium hydroxide (analytical grade), dissolve it with a small amount of purified water and transfer it to a 1000ml volumetric flask, then accurately measure 1ml of glacial acetic acid (analytical grade) and add Put it into a volumetric flask, dilute to the mark with purified water, shake well, and adjust the pH to 4.75 with glacial acetic acid (analytical grade).
[0051] 2) Extraction and separation of cephalosporin residue
[0052]Take the spray-dried and inactivated solid cephalosporin residue sample, grind it with...
Embodiment 2
[0083] Example 2 Determination of Cephalosporin C Residual Potency in Liquid Inactivated Cephalosporin Residue
[0084] 1) Preparation of buffer
[0085] With step 1) among the embodiment 1.
[0086] 2) Extraction and separation of cephalosporin residue
[0087] Take the liquid inactivated cephalosporin residue sample, and use a medium-strength grinding bowl for more than 300 laps to grind to a fine and uniform paste, so that the particle size is D97=33.20 μm; weigh 4 g of the liquid inactivated cephalosporin residue grinding sample, and put it in Into a 50ml polypropylene centrifuge tube, add 20ml of 0.1mol / L phosphate buffer (pH 5.0), vortex for 1min, ultrasonically assisted extraction for 30min, centrifuge at 4500rpm for 10min, and take the supernatant.
[0088] 3) Cephalosporin C residue titer detection
[0089] With step 3) in embodiment 1.
[0090] 4) Determination results of cephalosporin C residues in three batches of liquid inactivated cephalosporin residues
[0...
experiment example 1
[0096] Experimental example 1 Repeatability experiment
[0097] Weigh two parts of about 25 mg of cephalosporin C sodium salt reference substance, accurately weigh them, place them in two 100ml volumetric flasks respectively, dissolve and dilute to the mark with 0.01mol / L sodium acetate solution (pH4.75), weigh Spray inactivated cephalosporin residue grinding sample 2g, in a 50ml polypropylene centrifuge tube, add 20ml of 0.1mol / L phosphate buffer (pH 5.0), vortex for 1min, ultrasonically assisted extraction for 30min, centrifuge at 4500rpm for 10min, take supernatant. Take 0.5ml of the control solution and 1ml of the sample solution, mix and dilute to 10ml, repeat the measurement 5 times according to the chromatographic conditions of the analysis method, the results are shown in Table 3.
[0098] Table 3 Repeatability experiment
[0099] serial number
[0100] Conclusion: After 6 consecutive injections, the RSD of retention time was 0.05%, and the RSD of peak area...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com