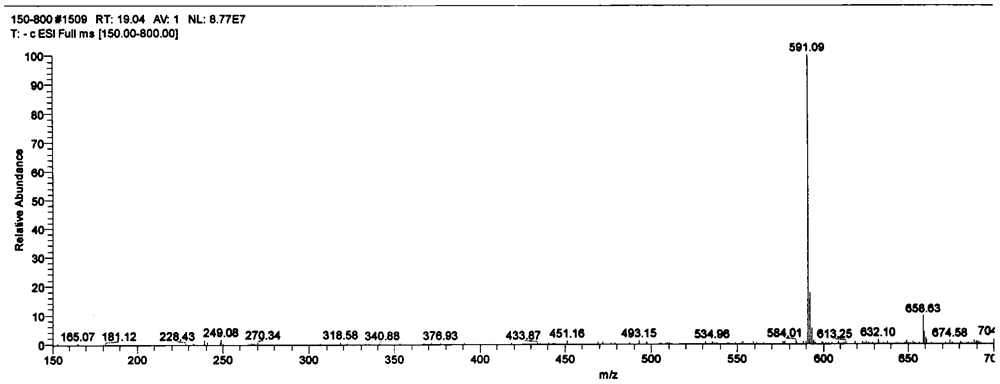
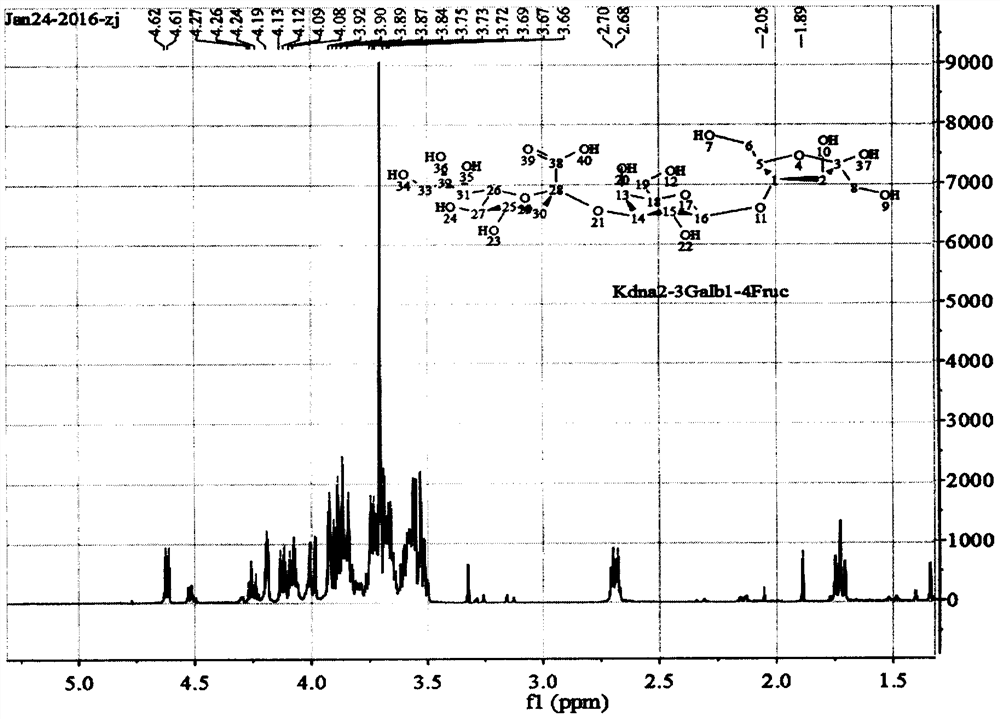
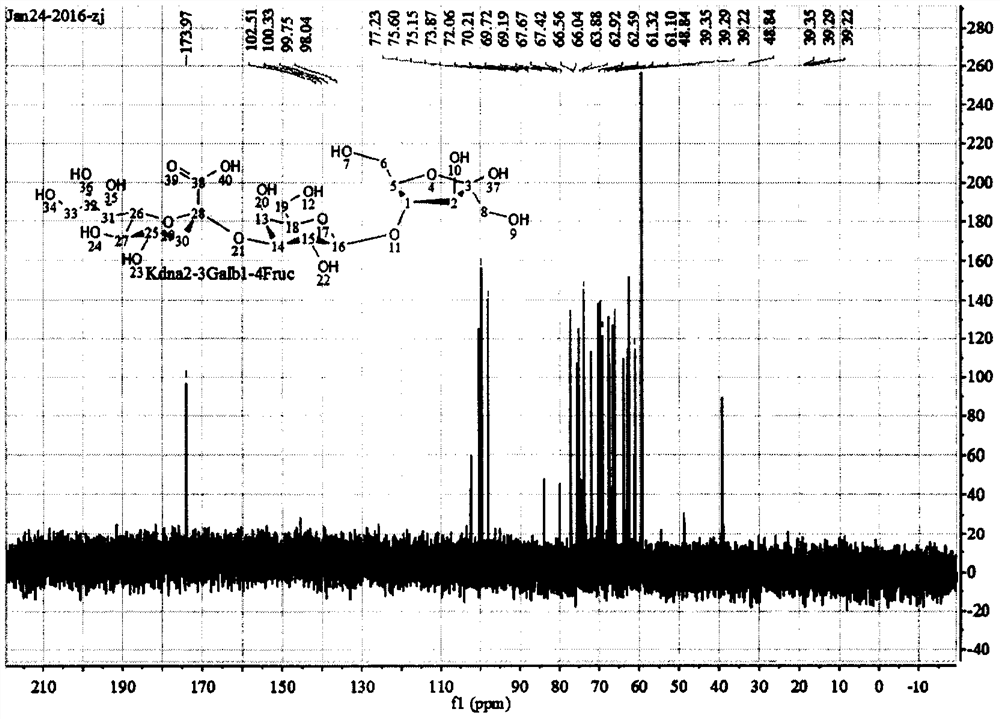
A kind of preparation method of α-2,3 deaminosialyllactulose
A technology of lactulose and sialic acid, applied in the field of α-2, can solve problems such as the difficulty of deaminosialic acid glycosylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] A method for preparing α-2,3 deaminosialyllactulose, characterized in that: 40 parts by weight of mannose, 100 parts by weight of sodium pyruvate, 50 parts by weight of lactulose, and 150 parts by weight of cytidine triphosphate sodium (CTP) , dissolved in 4000 parts by weight of ultrapure water, adjusted to pH 8.5 with 4 mol / liter of NaOH, adding 400 parts by weight of Tris-HCl of 1 mol / liter of pH 8.5; adding 40 parts by weight of 2 mol / liter of MgCl 2 ; Add 5 parts by weight of sialic acid aldolase, 6 parts by weight of CMP-sialyltransferase; 6 parts by weight of α-2,3 sialyltransferase. Then the mixture was placed in a 37°C air bath shaker and reacted for 24 hours. The degree of reaction was detected by silica gel thin layer chromatography (TLC), and the developing solvent of TLC was n-propanol:methanol:water=4:2:1. After the lactulose reaction is complete, add the same volume of 95% ethanol to the reaction solution, mix well, put it in a refrigerator at 4°C for 30...
Embodiment 2
[0016] A method for preparing α-2,3 deaminosialyllactulose, characterized in that: 80 parts by weight, 200 parts by weight of sodium pyruvate, 100 parts by weight of lactulose, 260 parts by weight of cytidine triphosphate sodium (CTP), dissolved in In 8000 parts by weight of ultrapure water, adjust the pH to 8.5 with 4 mol / liter of NaOH, add 1000 parts by weight of Tris-HCl of 1 mol / liter of pH 8.5; add 100 parts by weight of MgCl of 2 mol / liter 2 ; Add 8 parts by weight of sialic acid aldolase, 10 parts by weight of CMP-sialyltransferase; 10 parts by weight of α-2,3 sialyltransferase. Then the mixture was placed in a 37°C air bath shaker and reacted for 24 hours. The degree of reaction was detected by silica gel thin layer chromatography (TLC), and the developing solvent of TLC was n-propanol:methanol:water=4:2:1. After the lactulose reaction is complete, add the same volume of 95% ethanol to the reaction solution, mix well, put it in a refrigerator at 4°C for 30 minutes, th...
Embodiment 3
[0018] A method for preparing α-2,3 deaminosialyllactulose, characterized in that: 50 parts by weight of mannose, 120 parts by weight of sodium pyruvate, 65 parts by weight of lactulose, and 160 parts by weight of cytidine triphosphate sodium (CTP) , dissolved in 6000 parts by weight of ultrapure water, adjusted to pH 8.5 with 4 mol / liter of NaOH, adding 600 parts by weight of Tris-HCl of 1 mol / liter of pH 8.5; adding 60 parts by weight of 2 mol / liter of MgCl 2 ; Add 6 parts by weight of sialic acid aldolase, 7 parts by weight of CMP-sialyltransferase; 7 parts by weight of α-2,3 sialyltransferase. Then the mixture was placed in a 37°C air bath shaker and reacted for 24 hours. The degree of reaction was detected by silica gel thin layer chromatography (TLC), and the developing solvent of TLC was n-propanol:methanol:water=4:2:1. After the lactulose reaction is complete, add the same volume of 95% ethanol to the reaction solution, mix well, put it in a refrigerator at 4°C for 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com