Preparation method of 4-tertiary butyl calix(4)arene modified electrode applied to electrochemical method to recognize amino acid enantiomer
A technology of tert-butyl cup and modified electrode, which is applied in the fields of electrochemical analysis and biology, and achieves the effects of environmental protection and pollution-free preparation process, simple and easy method, and efficient identification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
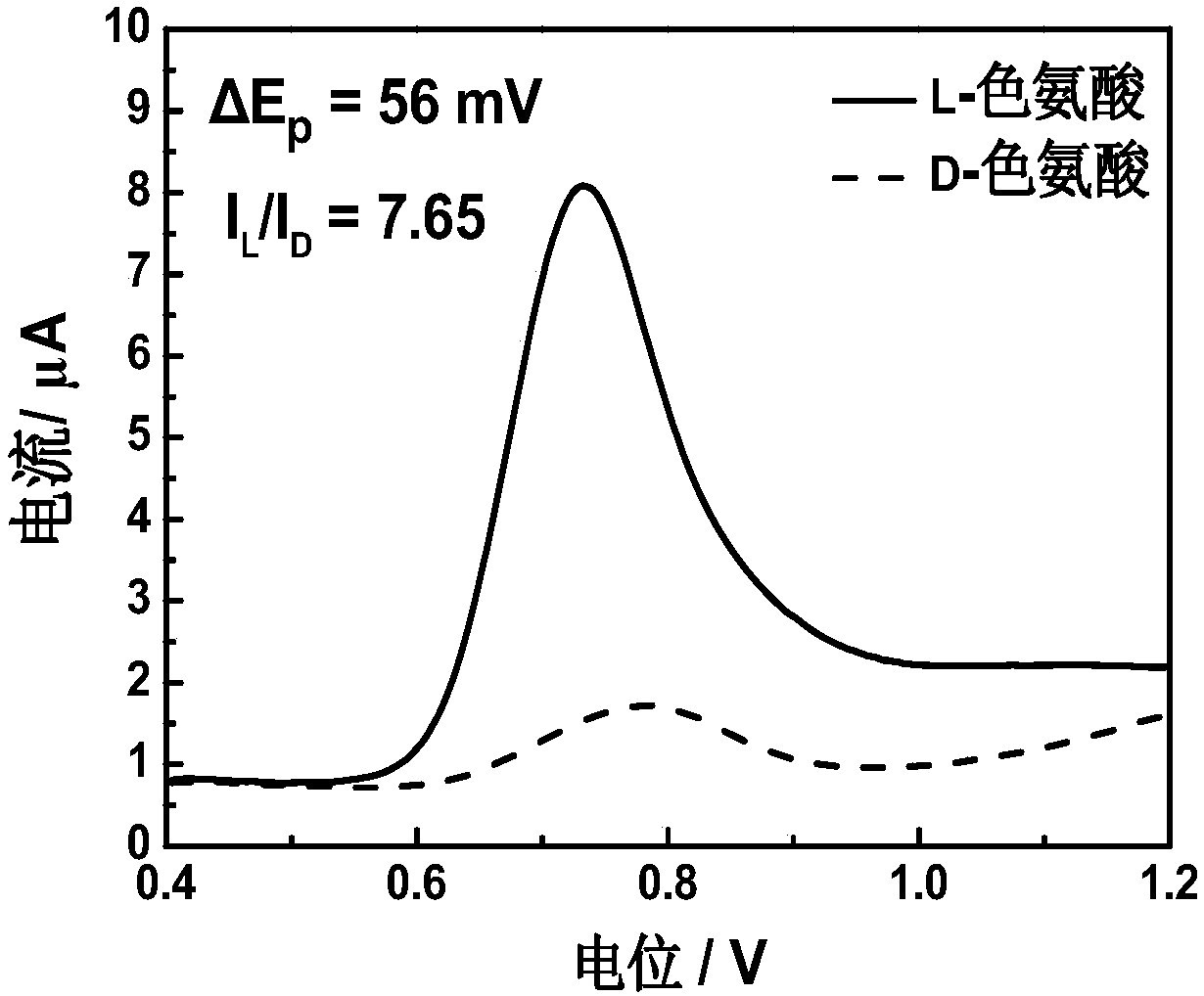
[0023] The preparation of 4-tert-butylcalix[4]arene modified electrode for electrochemical recognition of tryptophan enantiomers includes the following steps:
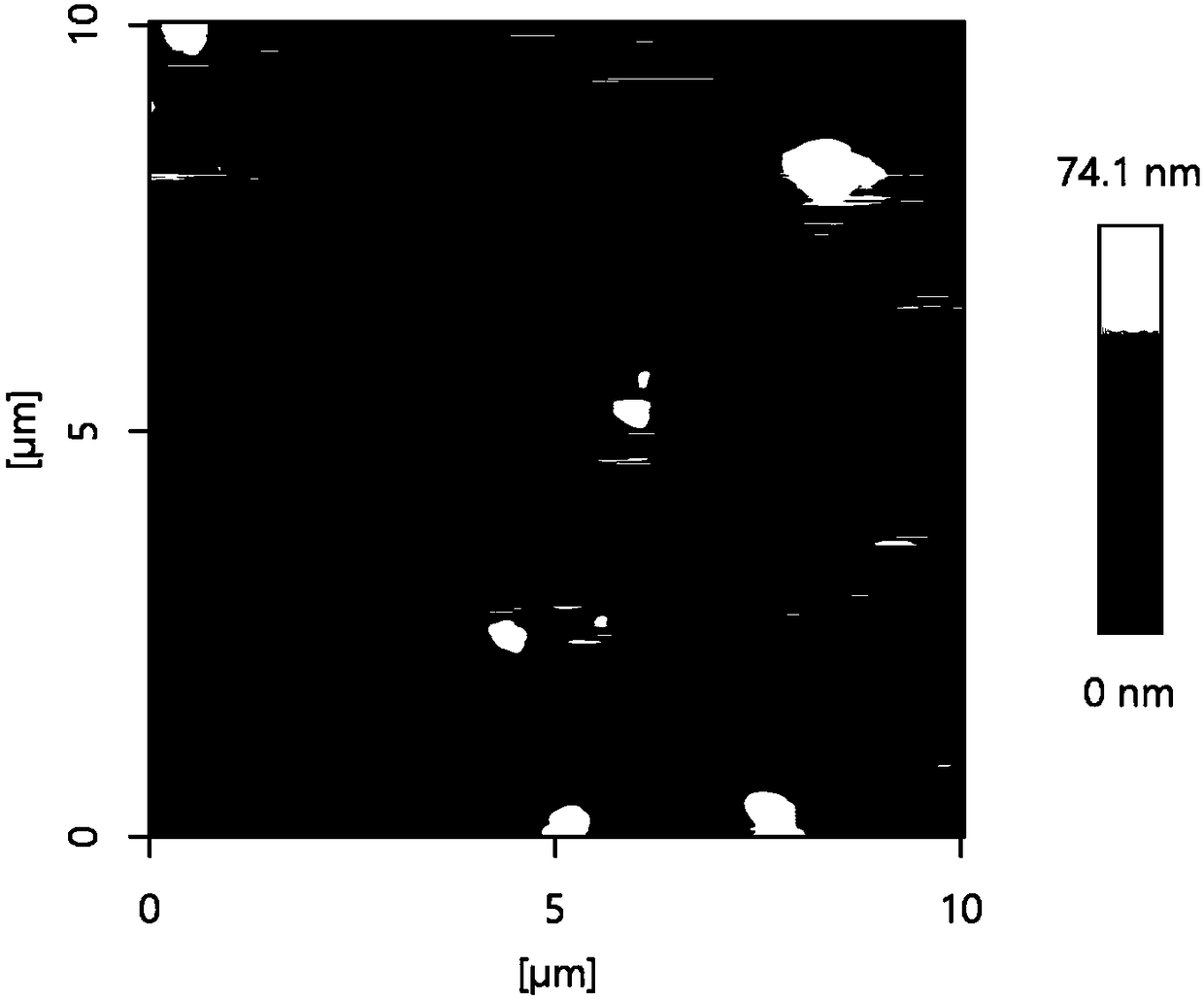
[0024] (1) Grinding 4-tert-butylcalix[4]arene into powder and ultrasonically dispersing it in ultrapure water to prepare a 3 mM 4-tert-butylcalix[4]arene dispersion. 10 μL was drip-coated on the surface of the glassy carbon electrode, and dried at room temperature to obtain a 4-tert-butylcalix[4]arene-modified electrode.
[0025] (2) A three-electrode system was used in the experiment, the 4-tert-butylcalix[4]arene modified electrode was used as the working electrode, the platinum plate electrode was used as the counter electrode, and the saturated calomel electrode was used as the reference electrode. The three-electrode system was respectively immersed in the prepared In 1.0mM L- / D-tryptophan solution, after standing still for 90s, within the potential range of 0.4-1.2V, conduct differential pulse test at a scan rate...
Embodiment 2
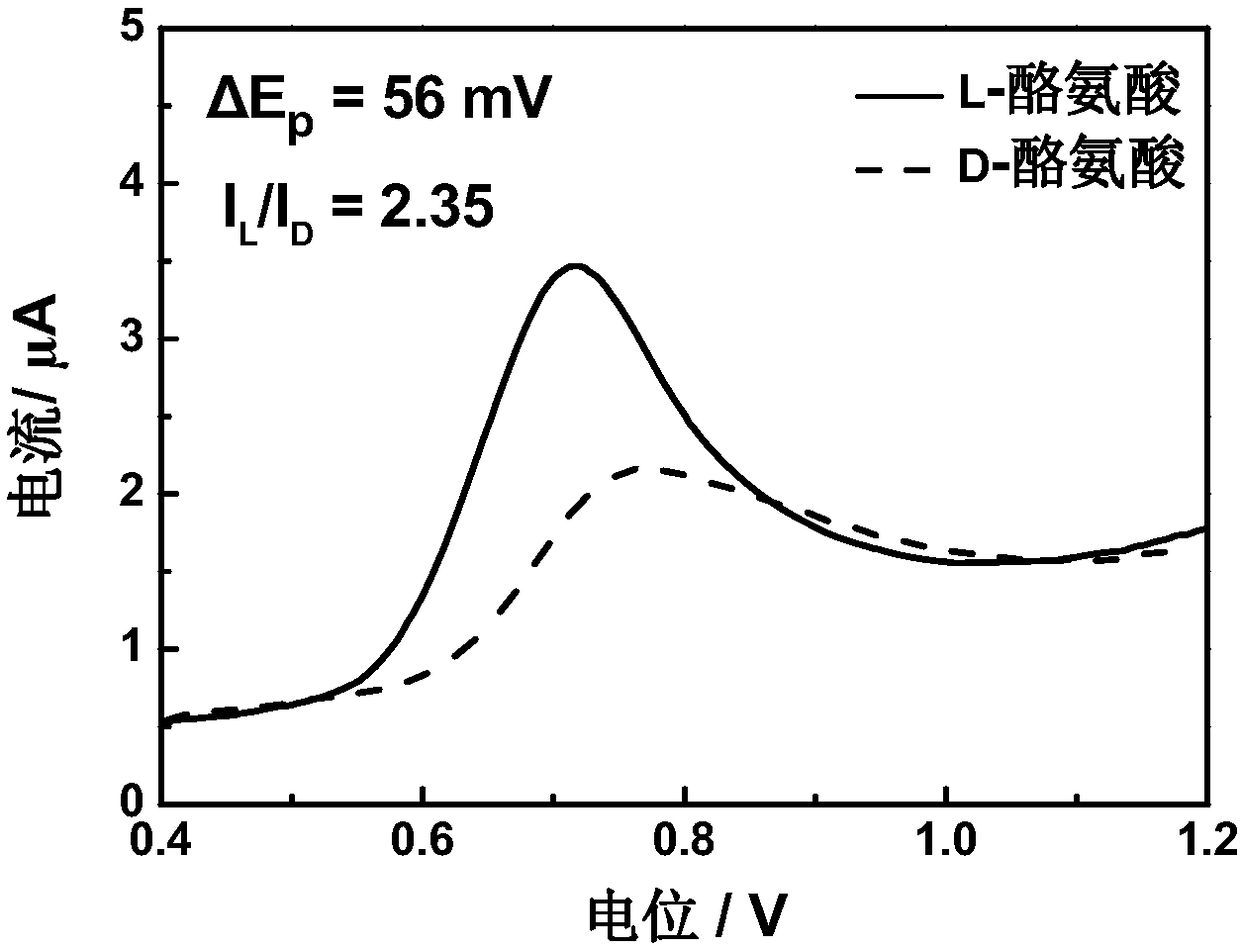
[0027] The preparation of 4-tert-butylcalix[4]arene modified electrode for electrochemical recognition of tyrosine enantiomers includes the following steps:
[0028] (1) Grinding 4-tert-butylcalix[4]arene into powder and ultrasonically dispersing it in ultrapure water to prepare a 3 mM 4-tert-butylcalix[4]arene dispersion. 10 μL was drip-coated on the surface of the glassy carbon electrode, and dried at room temperature to obtain a 4-tert-butylcalix[4]arene-modified electrode.
[0029] (2) A three-electrode system was used in the experiment, the 4-tert-butylcalix[4]arene modified electrode was used as the working electrode, the platinum plate electrode was used as the counter electrode, and the saturated calomel electrode was used as the reference electrode. The three-electrode system was respectively immersed in the prepared In 1.0mM L- / D-tyrosine solution, after standing still for 90s, in the potential range of 0.4-1.2V, conduct differential pulse test at a scan rate of 8mV / ...
Embodiment 3
[0031] Different concentrations of 4-tert-butylcalix[4]arene modified electrodes to identify tryptophan enantiomers include the following steps:
[0032] (1) Grind 4-tert-butylcalix[4]arene into powder and ultrasonically disperse it in ultrapure water to prepare 4-tert-butylcalix[4]arene dispersions with different concentrations. The experimental concentration is 1.0-5.0 mM. 10 μL of 4-tert-butylcalix[4]arene dispersion liquid with different concentrations was taken drop-coated on the surface of the glassy carbon electrode, and dried at room temperature to obtain a 4-tert-butylcalix[4]arene modified electrode.
[0033] (2) A three-electrode system was used in the experiment. The electrode modified with different concentrations of 4-tert-butylcalix[4]arene was used as the working electrode, the platinum plate electrode was used as the counter electrode, and the saturated calomel electrode was used as the reference electrode. The three-electrode system was respectively Immerse ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com