Method for preparing feed layer cells by using R6-MEF carried with Xist Tale suppression transcription factor R6
A R6-MEF, feeder cell technology, applied in genetically modified cells, biochemical equipment and methods, artificially induced pluripotent cells, etc., can solve cytoplasmic vacuoles, affect feeder layer quality, and change cell functions problems, to achieve the effect of good clone shape, high pluripotency gene expression level, and fast growth rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
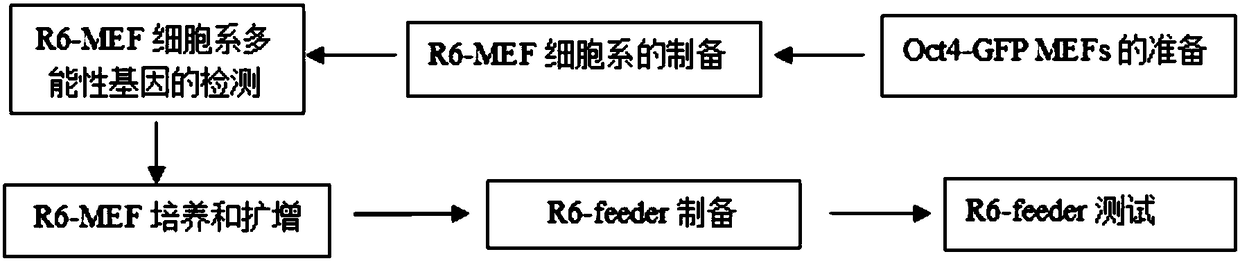
[0041] (2) Preparation of R6-MEF cell line:
[0042] A. Lipofection of Oct4-GFP MEFs:
[0043] Culture MEFs carrying Oct4-GFP in M10 culture medium in a 6-well plate, and transfect when Oct4-GFP MEFs reach 40%-60% confluence, replace with 2 mL of fresh M15 culture medium 30 minutes before transfection, put Equilibrate in the incubator for 30 minutes, add 500 μL transfection solution prepared and mixed in advance to the M15 culture medium, and place at 37°C, 5% CO 2 Cultivate in an incubator for liposome transfection for 12-18 hours;
[0044] B. Induction and establishment of R6-MEFs after liposome transfection:
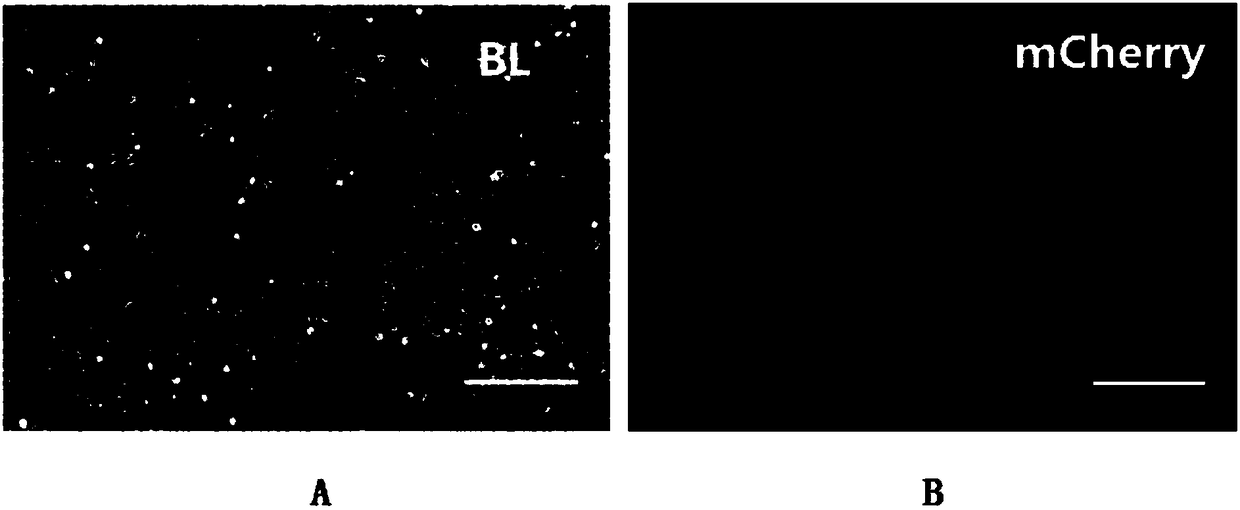
[0045] The next day after liposome transfection, replace the M15 culture medium with M15+Dox culture medium and culture at 37°C, 5% CO 2 Culture in an incubator; the third day after liposome transfection, the transfection efficiency was detected by fluorescence microscope: the successfully transfected cells expressed red fluorescent protein, replaced with new M15+D...
Embodiment 1
[0071] Embodiment 1: Preparation of Oct4-GFP MEFs
[0072] Sexually mature C57BL / 6J female mice and MF1, 129 / sv male mice carrying Oct4-GFP were caged together at a ratio of 1(♂):2(♀), and the fetuses were obtained by caesarean section from the 13.5-day-old pregnant mice and washed. After cleaning, remove the head, limbs and tail, wash and cut into pieces, digest with 0.25% Trypsin-EDTA digestion solution (25200056, Gibco) in a 37°C water bath for 20min, and mix several times during the period; continue to add 0.25% Trypsin-EDTA EDT A digestion solution (25200056, Gibco), digested in a water bath at 37°C for 20 minutes; pipetting and mixing, adding M10 culture medium to stop digestion, centrifuging at 1000rpm for 10 minutes, discarding the supernatant, adding an appropriate amount of M10 culture medium, repeating pipetting about 10 times, Place in a T25 culture flask at 37°C, 5% CO 2 Cultivate in an incubator, and after reaching 80% confluence, carry out 1:3-1:5 subculture an...
Embodiment 2
[0073] Embodiment 2: Preparation of R6-MEF cell line
[0074] A. Lipofection of Oct4-GFP MEFs:
[0075] Culture MEFs carrying Oct4-GFP in M10 culture medium in a 6-well plate, and transfect when Oct4-GFP MEFs reach 40%-60% confluence, replace with 2 mL of fresh M15 culture medium 30 minutes before transfection, put Equilibrate in the incubator for 30 minutes, add 500 μL transfection solution prepared and mixed in advance to the M15 culture medium, and place at 37°C, 5% CO 2 Cultivate in an incubator for liposome transfection for 12-18 hours;
[0076] B. Induction and establishment of R6-MEFs after liposome transfection:
[0077] On the second day after liposome transfection, replace the M15 culture medium with M15+Dox culture medium and culture in a 37°C, 5% CO2 incubator; on the third day after liposome transfection, detect the transfection efficiency with a fluorescence microscope : Successfully transfected cells express red fluorescent protein, replace with new M15+Dox+p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com