Rhodamine B modified semi-sandwich iridium complex with fluorescence characteristics as well as preparation method and application thereof
A technology of iridium complexes and fluorescent properties, applied in the field of chemical pharmaceuticals, can solve the problems of insufficient anti-cancer effects, unclear anti-cancer mechanisms, and unknown targets, and achieve excellent photophysical properties, photostability, and high yield , high anticancer activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Weigh 0.5g IrCl 3 ·nH 2 4 parts of O were added to 4 inner tanks of the microwave digestion apparatus respectively, and 0.75 mL of 1,2,3,4,5-pentamethylcyclopentadiene and 20 mL of methanol were added to each tank, ultrasonicated, nitrogen gas was passed, and the lids of the tanks were closed , Assemble the main tank and the standard tank, set the parameters of the microwave digestion instrument, react with the microwave digestion instrument, filter the obtained product, and remove the unreacted IrCl 3 ·nH 2 0, the liquid is spin-dried, and the solid obtained is dissolved with dichloromethane together with the crystal in the inner tank, and ether is added, and the single crystal is cultivated by the diffusion method to obtain the orange-yellow iridium dimer (R in the formula (1) A 1 = methyl) product, yield 51.4%.
Embodiment 2
[0046] Weigh 0.5g IrCl 3 ·nH 2 4 parts of O were added to 4 inner tanks of the microwave digestion apparatus respectively, and 1.3g of 1-biphenyl, 2,3,4,5-tetramethylcyclopentadiene, 20mL of methanol were added to each tank, ultrasonically, and nitrogen , cover the tank lid, assemble the main tank and the standard tank, set the parameters of the microwave digestion instrument, react with the microwave digestion instrument, filter the obtained product, and remove the unreacted IrCl 3 ·nH 2 O, the liquid is spin-dried, and the obtained solid is dissolved with dichloromethane together with the crystals in the inner tank, and ether is added, and the single crystal is cultivated by the diffusion method to obtain the orange-yellow iridium dimer (R in formula (1) A 1 = biphenyl) product, yield 20.6%.
Embodiment 3
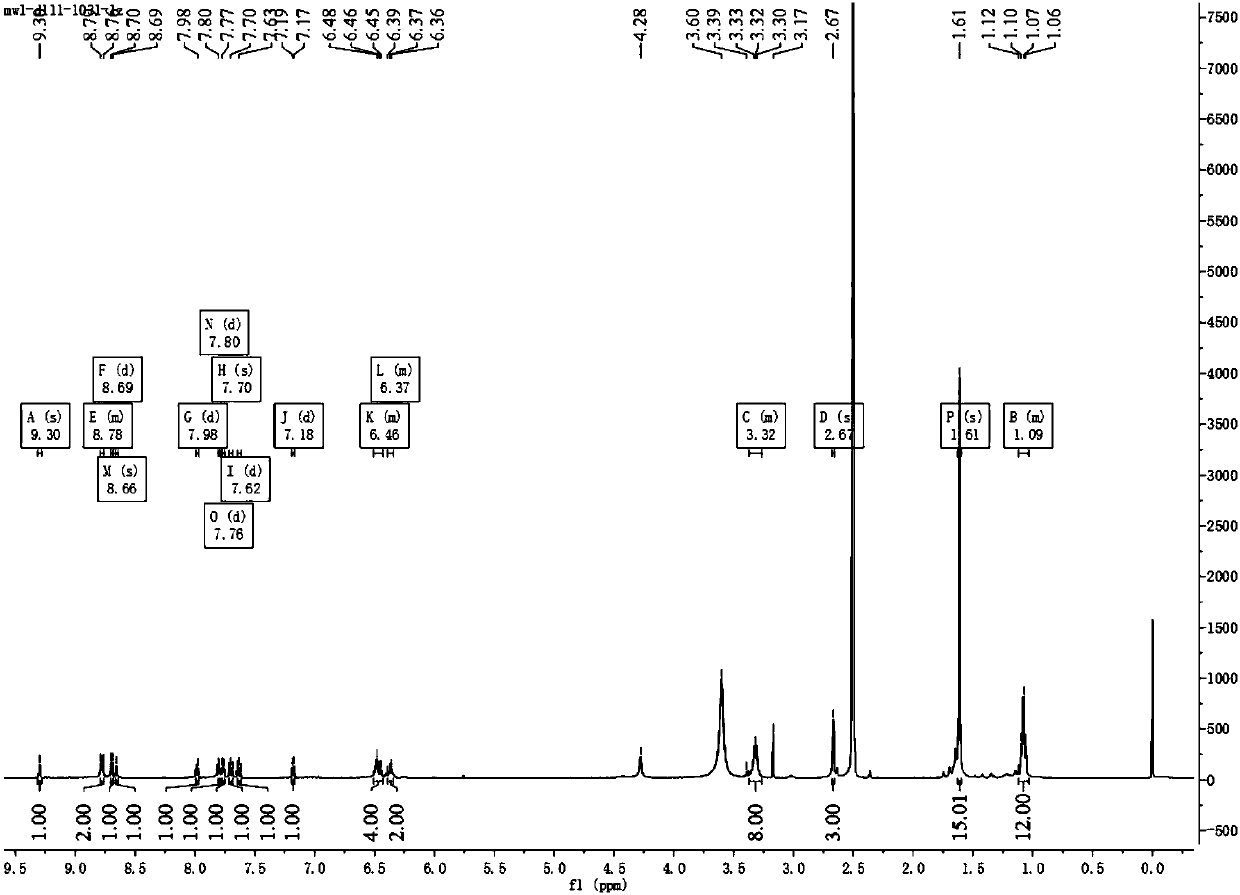
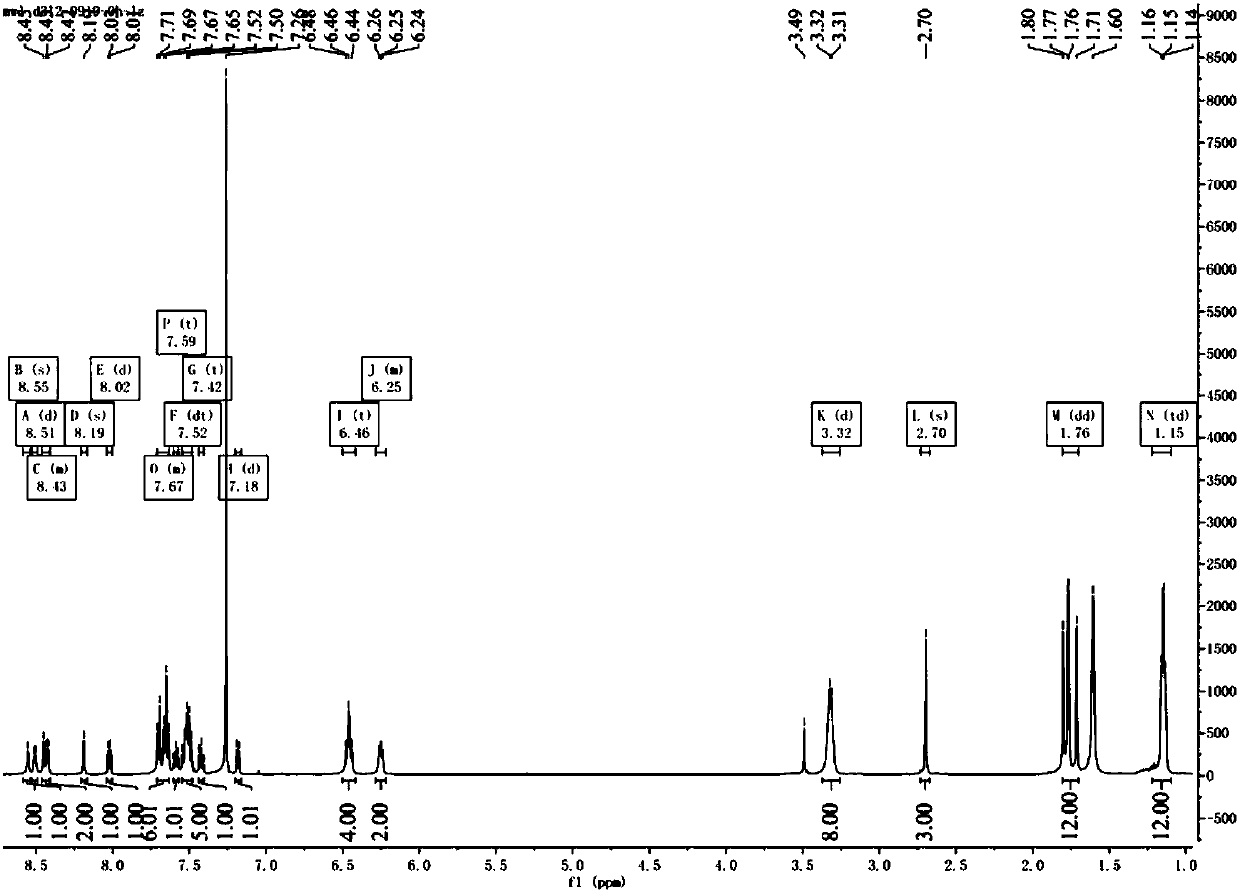
[0048] Synthesis of ligand L: Take 0.21g rhodamine B hydrazide and 0.10g 4-methyl-4'-formyl-2,2-bipyridine in a 100ml round bottom flask, add 30ml dichloromethane as solvent, add 1ml Formic acid, heat and reflux at 45°C for 24 hours, the solution turns from rose red to red, TCL detects the reaction process, new substances are produced, the liquid is spin-dried to red oil, add 1ml of methanol, put it in the refrigerator for a period of time, take it out , a beige solid was produced, suction filtered, the filter cake was washed several times with methanol, the filtrate was rose red, vacuum-dried, and the filter cake was taken to obtain a beige powder product with a yield of 49.67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com