A kind of nifedipine sustained-release tablet and preparation method thereof
A technology of nifedipine and sustained-release tablets, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as difficult bioequivalence and unstudied release behavior, and achieve The effect of increasing the dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
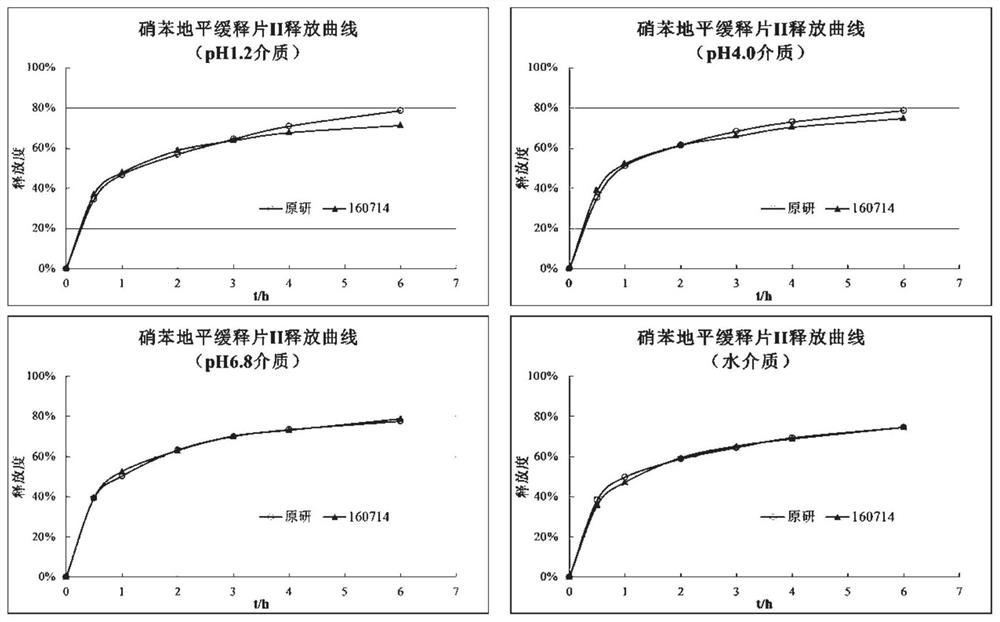
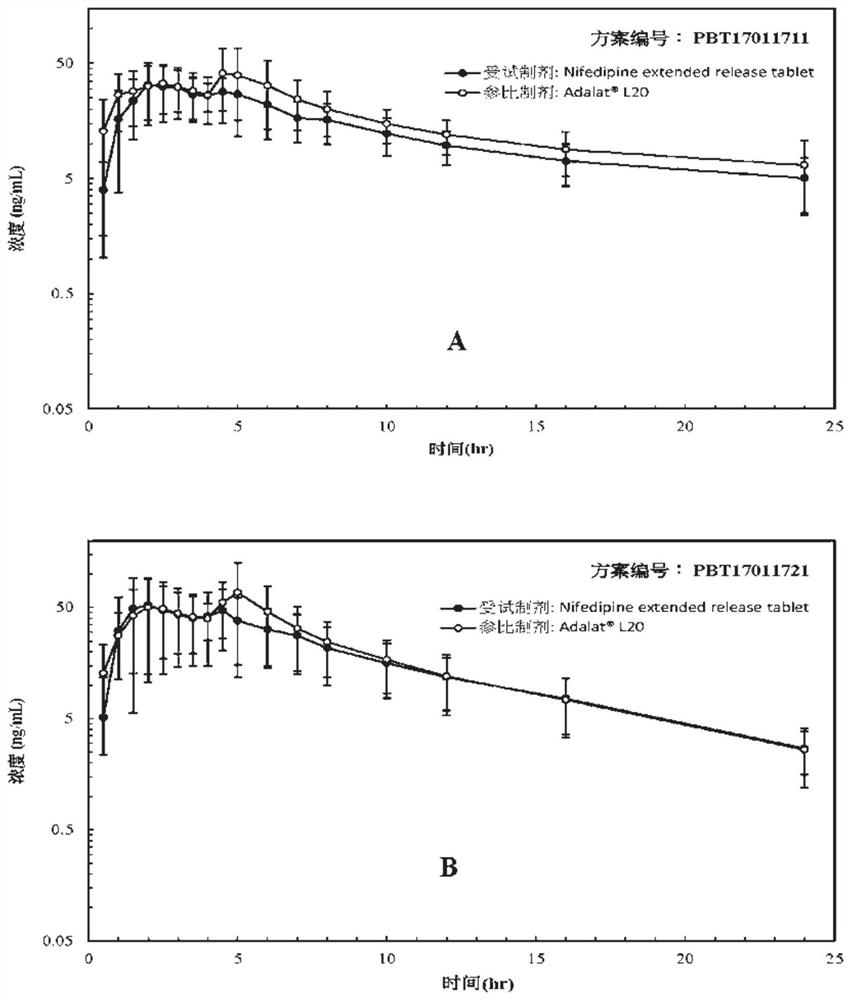
Examples
Embodiment 1
[0069] 20mg nifedipine sustained-release tablet prescription (300,000 / batch) and preparation method thereof:
[0070]
[0071] Preparation Process:
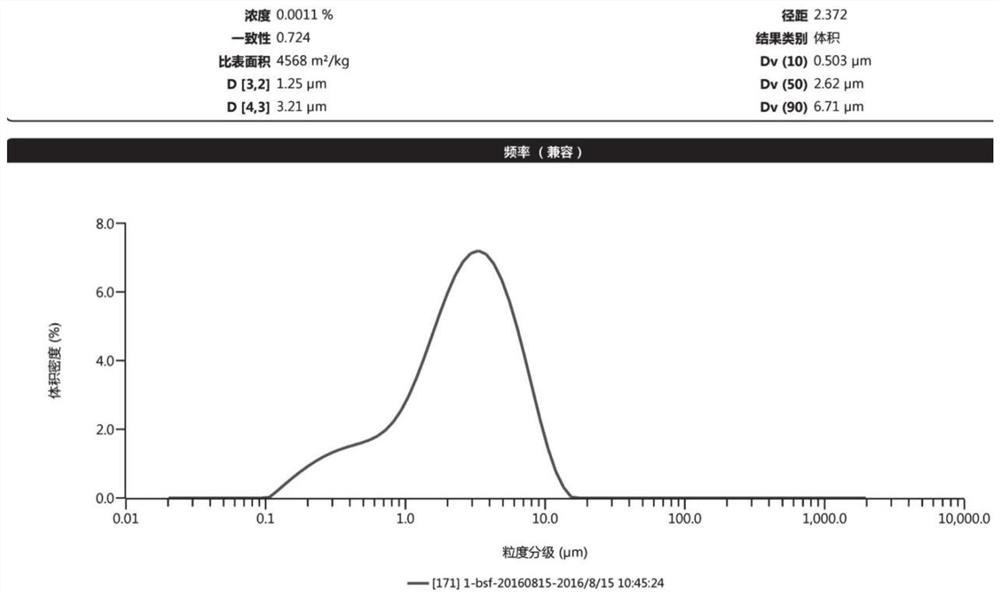
[0072] Pass the prescribed amount of microcrystalline cellulose, hypromellose, and hypromellose through an 80-mesh sieve, and control the particle size of ethyl cellulose to D 90 200 ~ 300um, standby; hypromellose, hypromellose, ethyl cellulose mixed uniformly as blocker composition; blocker composition, anhydrous calcium hydrogen phosphate and raw materials (D 90 5 ~ 10um) into the wet granulator, after stirring at speed I for 10 minutes, add microcrystalline cellulose and continue stirring for 5 minutes; add an appropriate amount of 95% ethanol to dissolve Tween 80, stir at speed I and chop at speed II during the liquid addition process 4min, out of the pan; after drying in an oven at 30°C for 1h, increase the temperature to 50°C and continue drying for 1h until the water content is lower than 3%, and granulate; add dry gr...
Embodiment 2
[0074] 20mg nifedipine sustained-release tablet prescription (300,000 / batch) and preparation method thereof:
[0075]
[0076] Preparation Process:
[0077] Pass the prescribed amount of microcrystalline cellulose, hypromellose, and hypromellose through an 80-mesh sieve, and control the particle size of ethyl cellulose to D 90 200 ~ 300um, standby; hypromellose, hypromellose, ethyl cellulose mixed uniformly as blocker composition; blocker composition, anhydrous calcium hydrogen phosphate and raw materials (D 90 5 ~ 10um) into the wet granulator, after stirring at speed I for 10 minutes, add microcrystalline cellulose and continue stirring for 5 minutes; add an appropriate amount of 95% ethanol to dissolve Tween 80, stir at speed I and chop at speed II during the liquid addition process 4min, out of the pan; after drying in an oven at 30°C for 1h, increase the temperature to 50°C and continue drying for 1h until the water content is lower than 3%, and granulate; add dry gr...
Embodiment 3
[0079] 20mg nifedipine sustained-release tablet prescription (300,000 / batch) and preparation method thereof:
[0080]
[0081] Preparation Process:
[0082] Pass the prescribed amount of microcrystalline cellulose, hypromellose, and hypromellose through an 80-mesh sieve, and control the particle size of ethyl cellulose to D 90200 ~ 300um, standby; hypromellose, hypromellose, ethyl cellulose mixed uniformly as blocker composition; blocker composition, anhydrous calcium hydrogen phosphate and raw materials (D 90 5 ~ 10um) into the wet granulator, after stirring at speed I for 10 minutes, add microcrystalline cellulose and continue stirring for 5 minutes; add an appropriate amount of 95% ethanol to dissolve Tween 80, stir at speed I and chop at speed II during the liquid addition process 4min, out of the pan; after drying in an oven at 30°C for 1h, increase the temperature to 50°C and continue drying for 1h until the water content is lower than 3%, and granulate; add dry gra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com