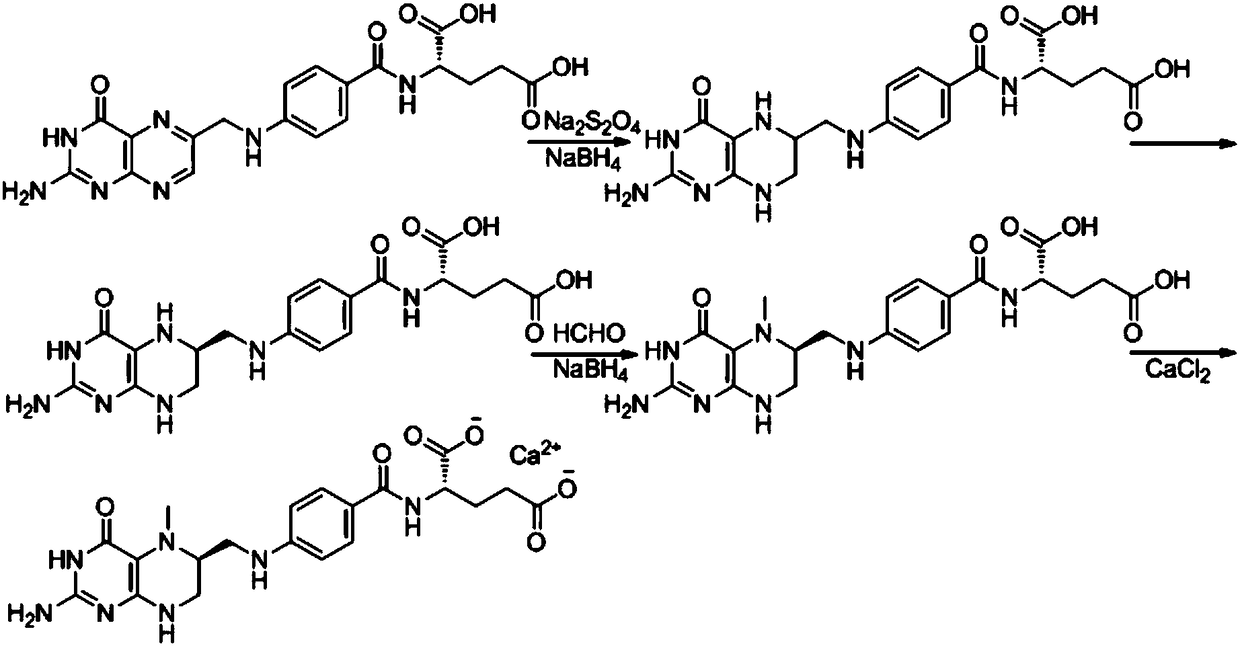
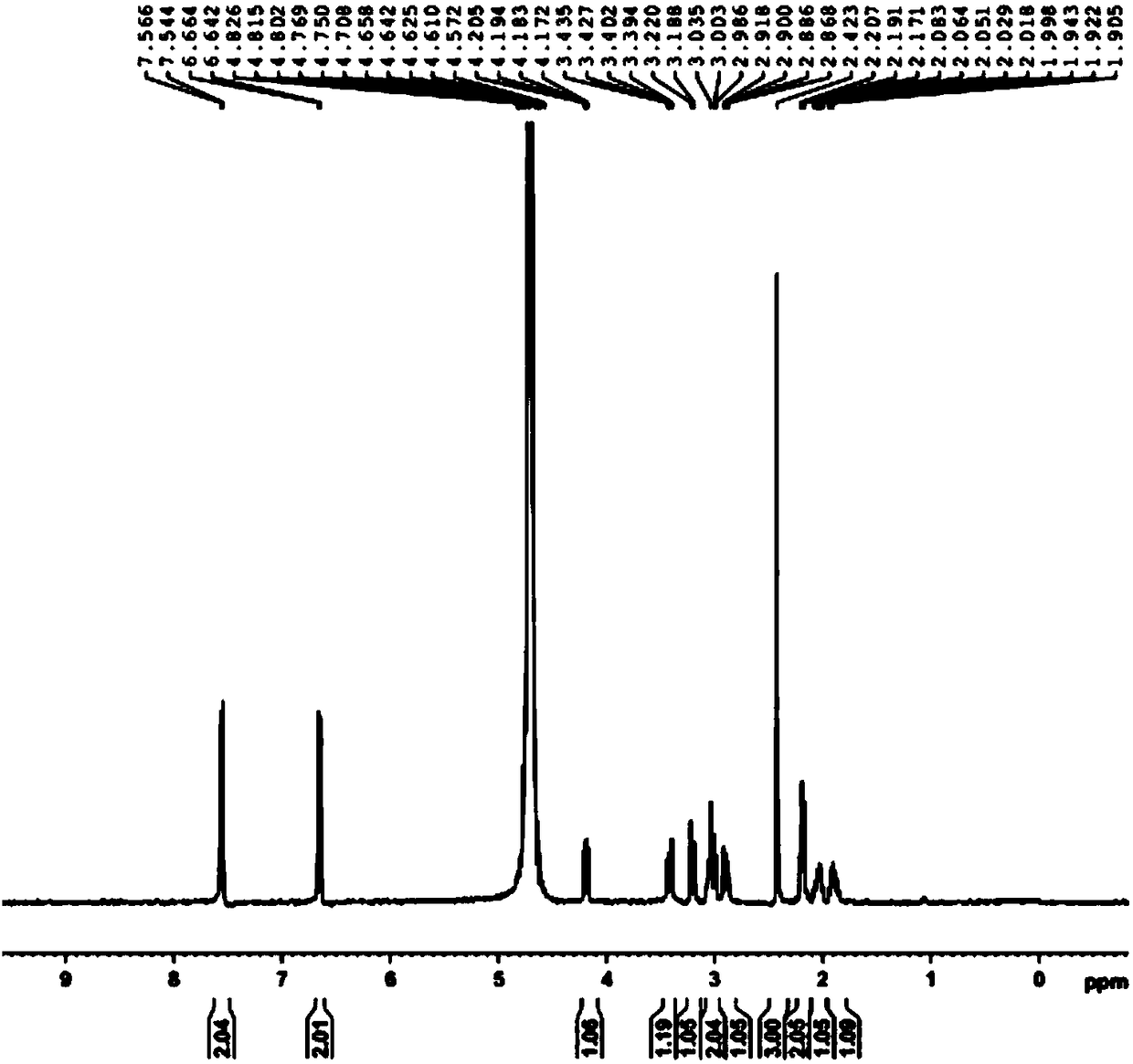
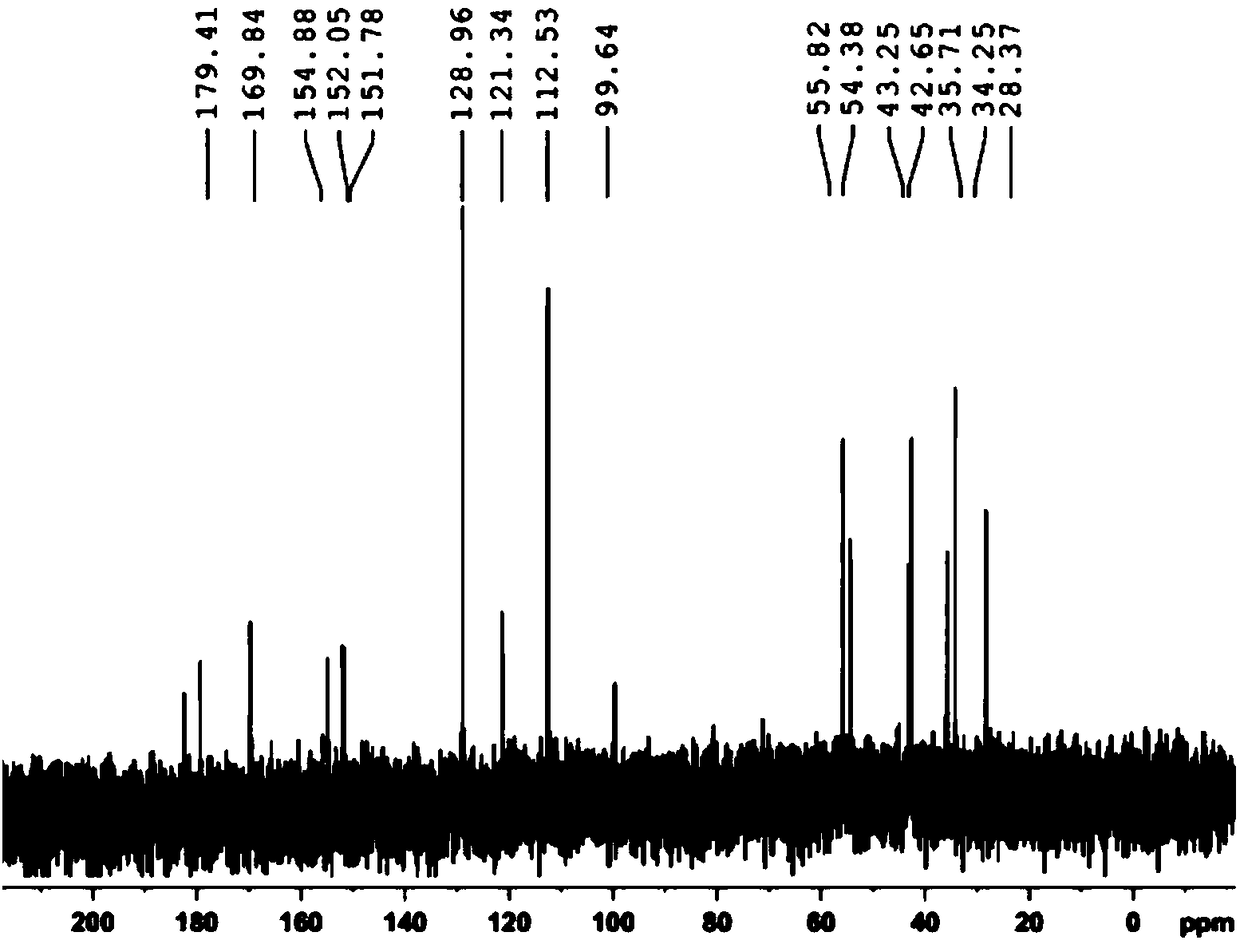
Preparation method of L-5-calcium methyltetrahydrofolate
A technology of calcium methyltetrahydrofolate and methyltetrahydrofolate, which is applied in the field of preparation of calcium L-5-methyltetrahydrofolate, can solve problems such as low purity, explosive hydrogen, and high temperature resistance, and achieve Reduce the cost of post-processing and hidden dangers of production safety, the synthesis steps are simple and easy, and the effect of reducing the amount of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A preparation method of L-5-methyltetrahydrofolate calcium, said preparation method comprising the steps of:
[0035] (1) Preparation of 6-(R,S)-tetrahydrofolate:
[0036] Suspend folic acid (200 g, 0.45 mol) in 1.0 L of purified water, add 100 ml of 30% aqueous sodium hydroxide solution until dissolved, pH = 7.9, under nitrogen protection, add sodium dithionite (157 g, 0.90 mol) in batches, Keep the reaction at 60°C for 1.5h, TLC detects that the folic acid disappears, and after cooling down to 50°C, slowly add the prepared sodium borohydride aqueous solution (34.5g, 0.91mol, dissolved in 70ml of purified water) dropwise, and control the internal temperature not to exceed 75°C, after the addition, keep the reaction at 75°C for 2 hours, cool down to below 15°C, quench with concentrated hydrochloric acid to pH = 6, add 2-mercaptoethanol (2ml), adjust the pH to about 3 with concentrated hydrochloric acid, and filter with suction. Washed twice with water and once with eth...
Embodiment 2
[0045] A preparation method of L-5-methyltetrahydrofolate calcium, said preparation method comprising the steps of:
[0046] (1) Preparation of 6-(R,S)-tetrahydrofolate:
[0047] Suspend folic acid (200 g, 0.45 mol) in 1.2 L of purified water, add 100 ml of 30% aqueous sodium hydroxide solution until dissolved, pH = 7.7, under nitrogen protection, add sodium dithionite (235 g, 1.35 mol) in batches, Keep the reaction at 65°C for 1.5h, TLC detects that the folic acid disappears, and after cooling down to 45°C, slowly add the prepared sodium borohydride aqueous solution (51g, 1.34mol, dissolved in 100ml purified water) dropwise, and control the internal temperature not to exceed 75°C ℃, after the addition, keep the reaction at 60℃ for 3h, cool down to below 15℃, quench with concentrated hydrochloric acid to pH = 7, add 2-mercaptoethanol (2ml), then adjust the pH to about 3 with concentrated hydrochloric acid, filter with suction and wash with water Twice, washed once with ethano...
Embodiment 3
[0055] A preparation method of L-5-methyltetrahydrofolate calcium, said preparation method comprising the steps of:
[0056] (1) Preparation of 6-(R,S)-tetrahydrofolate:
[0057]Suspend folic acid (200 g, 0.45 mol) in 1.4 L of purified water, add 100 ml of 30% aqueous sodium hydroxide solution until dissolved, pH = 7.6, under nitrogen protection, add sodium dithionite (390 g, 2.24 mol) in batches, Keep the reaction at 75°C for 1h, TLC detects that the folic acid disappears, and after cooling down to 45°C, slowly add the prepared sodium borohydride aqueous solution (85.5g, 2.25mol, dissolved in 170ml purified water) dropwise, and control the internal temperature not to exceed 75°C ℃, after the addition, keep the reaction at 75℃ for 2h, cool down to below 15℃, quench with concentrated hydrochloric acid to pH=6, add 2-mercaptoethanol (2ml), then adjust the pH to about 3 with concentrated hydrochloric acid, filter with suction and wash with water Twice, washed once with ethanol, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com